Abstract
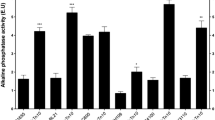
The phoE promoter region in Escherichia coli contains a −10 region, typical of σ70-dependent promoters and, instead of a normal −35 region, a so-called pho box, to which the transcriptional activator phospho-PhoB binds under low phosphate conditions. A second pho box is present upstream of the first one and is required for full expression of phoE during phosphate starvation. To determine whether the lack of expression under high phosphate conditions is due solely to the absence of a genuine −35 box, the −10 region was further optimized towards the consensus −10 sequence and promoter activity was measured using alkaline phosphatase as a reporter. The mutations resulted in a drastic increment in the basal level of expression under high phosphate conditions, indicating that the deviations from consensus in the −10 region also play a role in determining the poor expression of the wild-type promoter under these conditions. The expression under high phosphate conditions was partly dependent on the presence of the phoB gene, showing that a small amount of active PhoB must be present under these circumstances. During phosphate starvation, the activity of the mutant promoters was further induced. The upstream pho box was not required for full expression from the mutant promoters under these conditions. Apparently, the wild-type phoE promoter is carefully balanced by deviations from the optimal Pribnow box sequence that reduce expression under high phosphate conditions and by the presence of several copies of the pho box, which enhance expression under phosphate starvation.
Similar content being viewed by others
References
Agterberg M, Fransen R, Tommassen J (1988) Expression of Escherichia coli PhoE protein in avirulent Salmonella typhimurium aroA and galE strains. FEMS Microbiol Lett 50:295–299
Birnboim HC, Doty J (1979) A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res 7:1513–1524
Brickman E, Beckwith J (1975) Analysis of the regulation of Escherichia coli alkaline phosphatase synthesis using deletions and Φ80 transducing phages. J Mol Biol 96:307–316
Collado-Vides J, Magasanik B, Gralla JD (1991) Control site location and transcriptional regulation in Escherichia coli. Microbiol Rev 55:371–394
Cox GB, Webb D, Godovac-Zimmermann J, Rosenberg H (1988) Arg-220 of the PstA protein is required for phosphate transport through the phosphate-specific transport system in Escherichia coli but not for alkaline phosphatase repression. J Bacteriol 170:2283–2286
Cox GB, Webb D, Rosenberg H (1989) Specific amino acid residues in both the PstB and PstC proteins are required for phosphate transport by the Escherichia coli Pst system. J Bacteriol 171:1531–1534
Guan C, Wanner B, Inouye H (1983) Analysis of regulation of phoB expression using a phoB-cat fusion. J Bacteriol 156:710–717
Harley CB, Reynolds PR (1987) Analysis of Escherichia coli promoter sequences. Nucleic Acids Res 15:2343–2361
Hoffman CS, Wright A (1985) Fusions of secreted proteins to alkaline phosphatase: an approach for studying protein secretion. Proc Natl Acad Sci USA 82:5107–5111
Kasahara M, Makino K, Amemura M, Nakata A, Shinagawa H (1991) Dual regulation of the ugp operon by phosphate and carbon starvation at two interspaced promoters. J Bacteriol 173:549–558
Kim S, Makino K, Amemura M, Shinagawa H, Nakata A (1993) Molecular analysis of the phoH gene, belonging to the phosphate regulon in Escherichia coli. J Bacteriol 175:1316–1324
Kimura S, Makino K, Shinagawa H, Amemura M, Nakata A (1989) Regulation of the phosphate regulon of Escherichia coli: characterization of the promoter of the pstS gene. Mol Gen Genet 215:374–380
Lugtenberg B, Meijers J, Peters R, van der Hoek P, van Alphen L (1975) Electrophoretic resolution of the “major outer membrane” protein of Escherichia coli K12 into four bands. FEBS Lett 58:254–258
Makino K, Shinagawa H, Amemura M, Nakata A (1986) Nucleotide sequence of the phoB gene, the positive regulatory gene for the phosphate regulon of Escherichia coli K-12. J Mol Biol 190:37–44
Makino K, Shinagawa H, Amemura M, Kimura S, Nakata A, Ishihama A (1988) Regulation of the phosphate regulon of Escherichia coli. Activation of pstS transcription by PhoB protein in vitro. J Mol Biol 203:85–95
Makino K, Shinagawa H, Amemura M, Kawamoto T, Yamada M, Nakata A (1989) Signal transduction in the phosphate regulon of Escherichia coli involves phosphotransfer between PhoR and PhoB proteins. J Mol Biol 210:551–559
Makino K, Amemura M, Kim S, Nakata A, Shinagawa H (1993) Role of the σ70 subunit of RNA polymerase in transcriptional activation by activator protein PhoB in Escherichia coli. Genes Dev 7:149–160
McClure WR (1985) Mechanism and control of transcription initiation in prokaryotes. Annu Rev Biochem 54:171–204
O'Neill MC (1989) Escherichia coli promoters. J Biol Chem 264:5522–5530
Overbeeke N, Bergmans H, van Mansfeld F, Lugtenberg B (1983) Complete nucleotide sequence of phoE, the structural gene for the phosphate limitation inducible outer membrane pore protein of Escherichia coli K12. J Mol Biol 163:513–532
Overduin P, Boos W, Tommassen J (1988) Nucleotide sequence of the ugp genes of Escherichia coli K-12: homology to the maltose system. Mol Microbiol 2:767–775
Raibaud O, Schwartz M (1984) Positive control of transcription initiation in bacteria. Annu Rev Genet 18:173–206
Shuttleworth H, Taylor J, Minton N (1986) Sequence of the gene for alkaline phosphatase from Escherichia coli JM83. Nucleic Acids Res 14:8689
Struyvé M, Visser J, Adriaanse H, Benz R, Tommassen J (1993) Topology of the PhoE porin: the ‘eyelet’ region. Mol Microbiol 7:131–140
Surin BP, Jans DA, Fimmel AL, Shaw DC, Cox GB, Rosenberg H (1984) Structural gene for the phosphate-responsible phosphate-binding protein of Escherichia coli has its own promoter: complete nucleotide sequence of the phoS gene. J Bacteriol 157:772–778
Tautz D, Renz M (1983) An optimal freeze-squeeze method for the recovery of DNA fragments from agarose gels. Anal Biochem 132:14–19
Tommassen J, Lugtenberg B (1980) Outer membrane protein e of Escherichia coli K-12 is co-regulated with alkaline phosphatase. J Bacteriol 143:151–157
Tommassen J, van Tol H, Lugtenberg B (1983) The ultimate localization of an outer membrane protein of Escherichia coli K-12 is not determined by the signal sequence. EMBO J 2:1275–1279
Tommassen J, Koster M, Overduin P (1987) Molecular analysis of the promoter region of the Escherichia coli K-12 phoE gene. Identification of an element, upstream of the promoter, required for efficient expression of PhoE protein. J Mol Biol 198:633–641
Torriani A (1990) From cell membrane to nucleotides: the phosphate regulon in Escherichia coli. Bioessays 12:371–376
Werel W, Schickor P, Heumann H (1991) lexibility of the DNA enhances affinity of Escherichia coli RNA polymerase. EMBO J 10:2589–2594
Yamada M, Makino K, Amemura M, Shinagawa H, Nakata A (1989) Regulation of the phosphate regulon of Escherichia coli: analysis of mutant phoB and phoR genes causing different phenotypes. J Bacteriol 171:5601–5606
Author information
Authors and Affiliations
Additional information
Communicated by C. A. M. J. J. van den Hondel
Rights and permissions
About this article
Cite this article
Scholten, M., Tommassen, J. Effect of mutations in the −10 region of the phoE promoter in Escherichia coli on regulation of gene expression. Molec. Gen. Genet. 245, 218–223 (1994). https://doi.org/10.1007/BF00283270
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00283270