Abstract
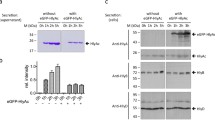
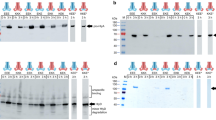
Secretion of Escherichia coli hemolysin is mediated by a sec-independent pathway which requires the products of at least three genes, hlyB, hlyD and tolC. Two regions of HlyD were studied. The first region (region A), consisting of the 33-amino acid, C-terminal part of the HlyD protein, is predicted to form a potential helix-loop-helix structure. This sequence is conserved among HlyD analogues of similar transport systems of other bacterial species. Using site-directed mutagenesis, we showed that the amino acids Leu475, Glu477 and Arg478 of this region are essential for HlyD function. The last amino acid of HlyD, Arg478, is possibly involved in the release of the HlyA protein, since cells bearing a hlyD gene mutant at this position produce similar amounts of HlyA to the wild-type strain, but most of the protein remains cell-associated. Competition experiments between wild-type and mutant HlyD proteins indicate that region A interacts directly with a component of the secretion apparatus. The second region of HIyD (region B), located between amino acids Leul27 and Leu170, is highly homologous to the otherwise unrelated outer membrane protein TolC. Deletion of this region abolishes secretion of hemolysin. This sequence of HlyD also seems to interact with a component of the hemolysin secretion machinery since a hybrid HIyD protein carrying the corresponding TolC sequence, although inactive in the transport of HlyA, is able to displace wild-type HlyD from the secretion apparatus.
Similar content being viewed by others
References
Benz R, Maier E, Gentschev I (1993) TolC of Escherichia coli functions as an outer membrane channel. Zentralbl Bakteriol 278:187–196
Braun V, Schönherr R, Hobbie S (1993) Enterobacterial hemolysins: activation, secretion and pore formation. Trends Microbiol 1:211–216
Devereux J, Haeberli P, Smithies O (1984) A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res 12:387–395
Fath MJ, Kolter R (1993) ABC transporters: bacterial exporters. Microbiol Rev 57:995–1017
Garnier J, Osguthorpe DJ, Robson B (1978) Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol 120:97–120
Gentschev I, Goebel W (1992) Topological and functional studies on HlyB of Escherichia coli. Mol Gen Genet 232:40–48
Gentschev I, Hess J, Goebel W (1990) Change in the cellular localization of alkaline phosphatase by alteration of its carboxyterminal sequence. Mol Gen Genet 222:211–216
Gray L, Baker K, Kenny B, Mackman N, Haigh R, Holland IB (1989) A novel C-terminal signal sequence targets Escherichia coli haemolysin directly to the medium. J Cell Sci Suppl 11:45–57
Hess J, Wels W, Vogel M, Goebel W (1986) Nucleotide sequence of a plasmid-encoded hemolysin determinant and its comparison with a corresponding chromosomal hemolysin sequence. FEMS Lett 34:1–11
Hess J, Gentschev I, Goebel W, Jarchau T (1990) Analysis of the haemolysin secretion system by PhoA-HlyA fusion proteins. Mol Gen Genet 224:201–208
Higgins CF (1989) Export-import family expands. Nature 340:342
Kenny B, Haigh R, Holland IB (1992) Identification of individual amino acids required for secretion within the haemolysin (HlyA) C-terminal targeting region. Mol Microbiol 6:1477–1489
Koronakis V, Koronakis E, Hughes C (1993) ATPase activity and ATP/ADP-induced conformational change in the soluble domain of the bacterial protein translocator HlyB. Mol Microbiol 8:1163–1175
Kramer W, Drutsa V, Jansen HW, Kramer B, Pflugfelder M, Fritz HJ (1984) The gapped duplex DNA approach to oligonucleotidedirected mutation construction. Nucleic Acids Res 12:9441–9456
Laemmli UK (1970) Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 227:680–685
Letoffe S, Ghigo J-M, Wandersman C (1993) Identification of two components of the Serratia marcescens metalloprotease transporter: protease SM secretion in Escherichia coli is TolC dependent. J Bacteriol 175:7321–7328
Ludwig A, Goebel W (1991) Genetic determinants in Gram-negative bacteria. In: Alouf JE, Freer JH (eds) Structure, regulation and activity of bacterial toxins. Academic Press, London, pp 117–146
Martinez E, Bartolomé B, de la Cruz F (1988) pACYC184-derived cloning vectors containing the multiple cloning site and lacZα reporter gene of pUC8/9 and pUC18/19 plasmids. Gene 68:159–162
Nicaud JM, Mackman N, Gray L, Holland IB (1986) The C-terminal 23 kD peptide of E. coli hemolysin 2001 contains all information necessary for its secretion by the hemolysin (hly) export machinery. FEBS Lett 204:331–335
Niki H, Imamura R, Ogura T, Hiraga S (1990) Nucleotide sequence of the tolC gene of Escherichia coli. Nucleic Acids Res 18:5547
Oropeza-Wekerle RL, Speth W, Imhof B, Gentschev I, Goebel W (1990) Translocation and compartmentalization of Escherichia coli hemolysin (HlyA). J Bacteriol 172:3711–3717
Perbal B (1988) A practical guide to molecular cloning. Wiley, New York
Pugsley AP (1993) The complete general secretory pathway in gram-negative bacteria. Microbiol Rev 57:50–108
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning, a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York
Sanger F, Nicklen S, Coulsen AR (1977) DNA sequencing with chaintermination inhibitors. Proc Natl Acad Sci USA 74:5463–5467
Schülein R, Gentschev I, Mollenkopf H-J, Goebel W (1992) A topological model for the haemolysin translocator protein HlyD. Mol Gen Genet 234:155–163
Stanley P, Koronakis V, Hughes C (1991) Mutational analysis supports a role for multiple structural features in the C-terminal secretion signal of Escherichia coli. Mol Microbiol 5:2931–2403
Stoker NG, Fairweather NF, Spratt BG (1982) Versatile low-copy number vectors for cloning in Escherichia coli. Gene 18:335–341
Vogel H, Jähnig F (1986) Models for the structure of outer membrane proteins of Escherichia coli derived from Raman spectroscopy and prediction methods. J Mol Biol 190:191–199
Wagner W, Vogel M, Goebel W (1983) Transport of hemolysin across the outer membrane of Escherichia coli requires two functions. J Bacteriol 154:200–210.
Wandersman C (1992) Secretion across the bacterial outer membrane. Trends Genet 8:317–322
Wandersman C, Delepelaire P (1990) TolC, an Escherichia coli outer membrane protein required for hemolysin secretion. Proc Natl Acad Sci USA 87:4776–4780
Wang R, Seror JS, Blight M, Pratt JM, Broome-Smith JK, Holland IB (1991) Analysis of the membrane organization of an Escherichia coli protein translocator, HlyB, a member of a large family of prokaryote and eukaryote surface transport proteins. J Mol Biol 217:441–454
Yanisch-Perron C, Vieira J, Messing J (1985) Improved M13 phage cloning vectors and host strains: nucleotide sequences and pUC19 vectors. Gene 33:103–119
Zhang F, Greig DI, Ling V (1993) Functional replacement of the hemolysin A transport signal by a different primary sequence. Proc Natl Acad Sci USA 90:4211–4215
Author information
Authors and Affiliations
Additional information
Communicated by E. Bautz
Rights and permissions
About this article
Cite this article
Schülein, R., Gentschev, I., Schlör, S. et al. Identification and characterization of two functional domains of the hemolysin translocator protein HlyD. Molec. Gen. Genet. 245, 203–211 (1994). https://doi.org/10.1007/BF00283268
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00283268