Summary
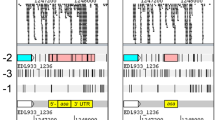
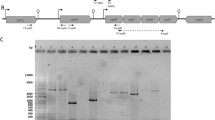
Homoprotocatechuate (HPC; 3,4-dihydroxyphenylacetate) is catabolized to Krebs cycle intermediates via extradiol (meta-) cleavage and the necessary enzymes are chromosomally encoded in a variety of bacteria. Based on an analysis of the cloned pathway genes, the Escherichia coli C hpc gene cluster was thought to be arranged in two gene blocks transcribed from a central, divergent, operator/promoter region, which was negatively regulated by the Hpc repressor. By a variety of techniques including expression of cloned hpc genes in pUC18/19 vectors, unidirectional deletion subcloning, hybridization studies and nucleotide sequencing it has now been shown that the hpc pathway structural genes are transcribed in one direction. These experiments have also indicated that a decarboxylase and an isomerase of the pathway are encoded by a single gene (hpcE) and have established the exact structural gene order as hpcRphpcECBDGH. The position of the putative regulatory gene, hpcR, is upstream of the first structural gene (hpcE) for the Hpc pathway enzymes. The deduced open reading frame for the Hpc repressor specifies a protein of 148 amino acids with a subunit molecular weight of 17 kDa. The region between hpcR and the first gene for the pathway enzymes has a sequence similar to that for catabolite activator protein (CAP) binding. This region is immediately upstream of a promoter for the pathway structural genes, which has been identified by transcript mapping.
Similar content being viewed by others
References
Assinder SJ, Williams PA (1990) The TOL plasmids: determinants of the catabolism of toluene and the xylenes. Adv Microbial Physiol 31:1–70
Boulnois GJ (1987) Gene cloning and analysis. A laboratory guide. Blackwell Scientific Publications, London
Boyd A, Kendall K, Simon MI (1983) Structure of the serine chemoreceptor in Escherichia coli. Nature 301:623–626
Burlingame R, Chapman PJ (1983) Catabolism of phenylpropionic acid and its 3-hydroxy derivative by Escherichia coli. J Bacteriol 155:113–121
Cooper RA, Skinner MA (1980) Catabolism of 3- and 4-hydroxyphenylacetate by the 3,4-dihydroxyphenylacetate pathway in Escherichia coli. J Bacteriol 143:302–306
Cooper RA, Jones DCN, Parrott S (1985) Isolation and mapping of Escherichia coli. K-12 mutants defective in phenylacetate degradation. J Gen Microbiol 131:2753–2757
Creighton TE (1970) N-(5′-phosphoribosyl) anthranilate isomeraseindol-3-ylglycerol phosphate synthetase of tryptophan biosynthesis. Relationship between the two activities of the enzyme for Escherichia coli. Biochem J 120:699–707
de Crombrugghe B, Busby S, Buc H (1984) Cyclic AMP receptor protein: role in transcriptional activation. Science 224:821–838
Fawcett T, Garrido-Pertierra A, Cooper RA (1989) 5-Carboxymethyl-2-hydroxymuconic semialdehyde dehydrogenases of Escherichia coli C and Klebsiella pneumoniae M5al show very high N-terminal sequence homology. FEMS Microbiol Lett 57:307–312
Ferrer E, Cooper RA (1988) Studies with a cloned Escherichia coli C 2-oxo-hept-3-ene-1,7-dioate hydratase gene. FEMS Microbiol Lett 52:155–160
Garrido-Pertierra A, Cooper RA (1981) Identification and purification of distinct isomerase and decarboxylase enzymes involved in the 4-hydroxyphenylacetate catabolic pathway of Escherichia coli. Eur J Biochem 117:581–584
Harayama S, Rekik M (1990) The meta cleavage operon of TOL degradative plasmid pWWO comprises 13 genes. Mol Gen Genet 221:113–120
Hareland WA, Crawford RL, Chapman PJ, Dagley S (1975) Metabolic function and properties of 4-hydroxyphenylacetic acid 1-hydroxylase from Pseudomonas acidovorans. J Bacteriol 121:272–285
Hawley DK, McClure D (1983) Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res 11:2237–2249
Hubacek J, Glover SW (1970) Complementation analysis of temperature-sensitive host specificity mutations in Escherichia coli. J Mol Biol 50:111–127
Jenkins JR (1987) Aromatic catabolism by Escherichia coli. PhD Thesis, University of Leicester, UK
Jenkins JR, Cooper RA (1988) Molecular cloning, expression and analysis of the genes of the homoprotocatechuate catabolic operon of Escherichia coli. C. J Bacteriol 170:5317–5324
Kraft R, Tardiff J, Krauter KS, Leinwant LA (1988) Using miniprep DNA for sequencing double stranded templates with Sequenase. Biotechniques 6:544–547
Kushner SR (1978) An improved method for the transformation of Escherichia coli with Colhl-derived plasmids. In: Boyer HB, Nicosia S (eds) Genetic engineering. Elsevier/North Holland, Amsterdam, pp 17–23
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning, a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York
Miller JH (1972) Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York
Parrott S, Jones S, Cooper RA (1987) 2-Phenylethylamine catabolism by Escherichia coli K-12. J Gen Microbiol 133:347–351
Roper DI, Cooper RA (1990a) Purification, some properties and nucleotide sequence of 5-carboxymethyl-2-hydroxymuconate isomerase of Escherichia coli C. FEBS Lett 266:63–66
Roper DI, Cooper RA (1990b) Purification, some properties and nucleotide sequence of homoprotocatechuate dioxygenase of Escherichia coli C. FEBS Lett 275:53–57
Skinner MA, Cooper RA (1982) An Escherichia coli mutant defective in the NAD-dependent succinate semialdehyde dehydrogenase. Arch Microbiol 132:270–275
Southern E (1979) Gel electrophoresis of restriction fragments. Methods Enzymol 68:152–176
Tabor S, Richardson CC (1987) DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci USA 84:4767–4771
Author information
Authors and Affiliations
Additional information
Communicated by J. Lengeler
Rights and permissions
About this article
Cite this article
Roper, D.I., Fawcett, T. & Cooper, R.A. The Escherichia coli C homoprotocatechuate degradative operon: hpc gene order, direction of transcription and control of expression. Molec. Gen. Genet. 237, 241–250 (1993). https://doi.org/10.1007/BF00282806
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00282806