Summary
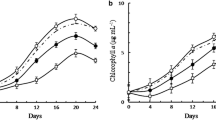
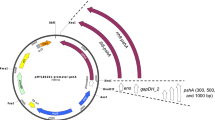
It has been suggested that a calcium-dependent intracellular protease of the cyanobacterium, Anabaena sp., participates in the differentiation of heterocysts, cells that are specialized for fixation of N2. Clones of the structural gene (designated prcA) for this protease from Anabaena variabilis strain ATCC 29413 and Anabaena sp. strain PCC 7120 were identified via their expression in Escherichia coli. The prcA gene from A. variabilis was sequenced. The genes of both strains, mutated by insertion of a drug resistance cassette, were returned to these same strains of Anabaena on suicide plasmids. The method of sacB-mediated positive selection for double recombinants was used to achieve replacement of the wild-type prcA genes by the mutated forms. The resulting mutants, which lacked Ca2+-dependent protease activity, were not impaired in heterocyst formation and grew on N2 as sole nitrogen source.
Similar content being viewed by others
References
Allen MM, Smith AJ (1969) Nitrogen chlorosis in blue-green algae. Arch Mikrobiol 69:114–120
Balbás P, Soberón X, Merino E, Zurita M, Lomeli H, Valle F, Flores N, Bolivar F (1986) Plasmid vector pBR322 and its special-purpose derivatives — a review. Gene 50:3–40
Bancroft I, Wolk CP, Oren EV (1989) Physical and genetic maps of the genome of the heterocyst-forming cyanobacterium Anabaena sp. strain PCC 7120. J Bacteriol 171:5940–5948
Bensadoun A, Weinstein D (1976) Assay of proteins in the presence of interfering materials. Anal Biochem 70:241–250
Brown RE, Jarvis KL, Hyland KJ (1989) Protein measurement using bicinchoninic acid: Elimination of interfering substances. Anal Biochem 180:136–139
Cai Y, Welk CP (1990) Use of a conditionally lethal gene in Anabaena sp. strain PCC 7120 to select for double recombinants and to entrap insertion sequences. J Bacteriol 170:3138–3145
Currier TC, Wolk CP (1979) Characteristics of Anabaena variabilis influencing plaque formation by cyanophage N-1. J Bacteriol 139:88–92
Elhai J, Wolk CP (1988a) Conjugal transfer of DNA to cyanobacteria. Methods Enzymol 167:747–754
Elhai J, Wolk CP (1988b) A versatile class of positive selection vectors based on the nonviability of palindrome-containing plasmids that allows cloning into long polylinkers. Gene 68:119–138
Fleming H, Haselkorn R (1974) The program of protein synthesis during heterocyst differentiation in nitrogen-fixing blue-green algae. Cell 3:159–170
Fogg GE (1949) Growth and heterocyst production in Anabaena cylindrica Lemm. II. In relation to carbon and nitrogen metabolism. Ann Bot [New Ser] 13:241–259
Foulds IJ, Carr NG (1977) A proteolytic enzyme degrading phycocyanin. FEMS Microbiol Lett 2:117–119
Frazier WC (1926) A method for the detection of changes in gelatine due to bacteria. J Infect Dis 39:302–309
Gangola P, Rosen BP (1987) Maintenance of intracellular calcium in Eseherichia coli. J Biol Chem 262:12570–12574
Gough JA, Murray NE (1983) Sequence diversity among related genes for recognition of specific targets in DNA molecules. J Mol Biol 166:1–19
Hanahan D (1985) Techniques for transformation of E. coli. In: Glover DM (ed) DNA cloning, vol. I. IRL Press, Oxford, pp 109–135
Haselkorn R (1978) Heterocysts. Annu Rev Plant Physiol 29:319–344
Haselkorn R, Mazur B, Orr J, Rice D, Wood N, Rippka R (1980) Heterocyst differentiation and nitrogen fixation in cyanobacteria (blue-green algae). In: Newton WE, Orme-Johnson WH (eds) Nitrogen fixation, vol II. University Park Press, Baltimore, pp 259–278
Herrero A, Wolk CP (1986) Genetic mapping of the chromosome of the cyanobacterium Anabaena variabilis. J Biol Chem 261:7743–7754
Hörger S (1988) Phycobilisomen-Abbau bei Stickstoffmangel: Vergleichende Untersuchungen in vivo und in vitro bei drei Cyanobakterien. Diploma thesis, University of Regensburg
Horinouchi S, Weisblum B (1982) Nucleotide sequence and functional map of pE194, a plasmid that specifies inducible resistance to macrolide, lincosamide, and streptogramin type B antibiotics. J Bacteriol 150:804–814
Hurn BAL, Chantler SM (1980) Production of reagent antibodies. Methods Enzymol 70:104–142
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lockau W, Massalsky B, Dirmeier A (1988) Purification and partial characterization of a calcium-stimulated protease from the cyanobacterium, Anabaena variabilis. Eur J Biochem 172:433–438
Mackinney G (1941) Absorption of light by chlorophyll solutions. J Biol Chem 140:315–322
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY
Marsh JL, Erfle M, Wykes EJ (1984) The pIC plasmid and phage vectors with versatile cloning sites for recombinant selection by insertional inactivation. Gene 32:481–485
Ownby JD, Shannahan M, Hood E (1979) Protein synthesis and degradation in Anabaena during nitrogen starvation. J Gen Microbiol 110:255–261
Ried JL, Collmer A (1987) An nptI-sacB-sacR cartridge for constructing directed, unmarked mutations in Gram-negative bacteria by marker exchange-eviction mutagenesis. Gene 57:239–246
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74:5463–5467
Sasagawa T, Ericssen LH, Walsh KA, Schreiber WE, Fischer EH, Titani K (1982) Complete amino acid sequence of human brain calmodulin. Biochemistry 21:2565–2569
Shine J, Dalgarno L (1974) The 3′-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci USA 71:1342–1346
Steinmetz M, Le Coq D, Aymerich S, Gonzy-Treboul G, Gay P (1985) The DNA sequence of the gene for the secreted Bacillus subtilis enzyme levansucrase and its genetic control sites. Mol Gen Genet 200:220–228
Thiel T (1984) Protein turnover during nitrogen and carbon starvation in the cyanobacterium Anabaena variabilis. Fifth International Symposium on Photosynthetic Procaryotes. Grindelwald, Switzerland, Abstr. VI 6
Thiel T, Wolk CP (1987) Conjugal transfer of plasmids to cyanobacteria. Methods Enzymol 153:232–243
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76:4350–4354
Trieu-Coot P, Carlier C, Martin P, Courvalin P (1987) Plasmid transfer by conjugation from Escherichia coli to Gram-positive bacteria. FEMS Microbiol Lett 48:289–294
Wolk CP (1982) Heterocysts. In: Carr NG, Whitton BA (eds) The biology of cyanobacteria. Blackwell, Oxford, pp 359–386
Wolk CP, Vonshak A, Kehoe P, Elhai J (1984) Construction of shuttle vectors capable of conjugative transfer from Escherichia coli to nitrogen-fixing filamentous cyanobacteria. Proc Natl Acad Sci USA 81:1561–1565
Wood NB, Haselkorn R (1979) Proteinase activity during heterocyst differentiation in nitrogen-fixing cyanobacteria. In: Cohen GN, Holzer H (eds) Limited proteolysis in microorganisms. US Department of Health, Education and Welfare Publication No. (NIH) 79-1591, US Government Printing Office, Washington DC, pp 159–166
Wood NB, Haselkorn R (1980) Control of phycobiliprotein proteolysis and heterocyst differentiation in Anabaena. J Bacteriol 141:1375–1385
Author information
Authors and Affiliations
Additional information
Communicated by H. Hennecke
Rights and permissions
About this article
Cite this article
Maldener, I., Lockau, W., Cai, Y. et al. Calcium-dependent protease of the cyanobacterium Anabaena: molecular cloning and expression of the gene in Escherichia coli, sequencing and site-directed mutagenesis. Molec. Gen. Genet. 225, 113–120 (1991). https://doi.org/10.1007/BF00282649
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00282649