Summary
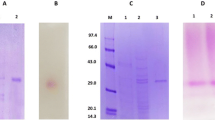
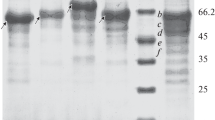
One invertase (Inv), five exoinulinases (Exo I; II; III; IV; V) and three endoinulinases (Endo I; II; III) were isolated from a commercial inulinase preparation derived from Aspergillus ficuum using ammonium sulfate precipitation, ion exchange chromatography on DEAE-Sephacel and DEAE-Trisacryl, gel filtration on Ultrogel and Fast Protein Liquid Chromatography on a Mono Q column. The invertase (Inv) had a molecular weight of 84000 and was much more active on sucrose than on inulin: the ratio of activity on inulin and sucrose (I/S ratio) was 0.01. The five exoinulinases show the same molecular weight of 74000 and I/S ratios in the range 0.16–0.36. The three endoinulinases had molecular weight of 64000 and I/S ratios in the range 0.86–2.92. All the β-fructofuranosidases were glycoproteins with a high sugar content (from 22 to 41% w/w). A. ficuum is the first described organism containing the three activities: invertase, exo and endoinulinase.
Similar content being viewed by others
References
Beluche I, Guiraud JP, Galzy P (1980) Inulinase activity of Debaromyces cantarellii. Folia Microbiol 25:32–39
Dedonder R (1952) Les glucides du topinambour. Bull Soc Chim Biol 34:157–172
Demeule S, Guiraud JP, Galzy P (1981) Study of Inulinase from Debaromyces phaffii capriotti. Z Allg Mikrobiol 21:181–189
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Calorimetric method for determination of sugars and related substances. Anal Biochem 28:350–356
Edelman J, Jefford TG (1964) The metabolism of fructose polymers in plants 4-β fructofuranosidases of tubers of Heliantus tuberosus L. Biochem J 93:148–161
Flood AE, Rutherford PP, Weston EW (1967) Effects of 2:4-dichlorophenoxyacetic Acid on enzyme systems in Jerusalem Artichoke tubers and Chicory roots. Nature 214:1049
Grootwassink JWD, Fleming SE (1980) Non specific β-fructofuranosidase (Inulinase) from Kluyveromyces fragilis batch and continuous fermentation, simple recovery method and some industrial properties. Enz Microb Technol 2:45–53
Guiraud JP (1981) Utilisation des levures pour la valorisation industrielle des polyfructosanes de type Inuline. Doctoral thesis, University of Montpellier, France
Guiraud JP, Viard-Gaudin C, Galzy P (1980) Etude de l'Inulinase de Candida salmenticensis Van Uden et Buckley. Agric Biol Chem 44:1245–1252
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lowry OH, Rosebrough NH, Farr AL, Randall RJ (1951) Protein estimation with Folin phenol reagent. J Biol Chem 193:265–275
Nahm BH, Byun SM (1977) Purification and characterization of Inulase from Kluyveromyces fragilis. Korean Biochem J 10:95–108
Nakamura T, Nakatsu S (1977) General properties of extracellular Inulase from Penicillium (studies of Microbial Inulase Part II). Nippon Nogei Kagaku Kaishi 51:681–689
Nakamura T, Kurokawa T, Nakatsu S, Ueda S (1978) Cristallisation and general properties of an extracellular inulinase. Nippon Nogei Kagaku Kaishi 52:159–166
Nakamura T, Maruki S, Nakatsu S, Ueda S (1978) General properties of Extracellular Inulinase (P II) from Aspergillus sp. (studies on microbial Inulase part II). Nippon Nogei Kagaku Kaishi 52:581–587
Negoro H (1973) Purification and enzymatic properties of extracellular β-fructofuranosidase from Candida Kefyr. J Ferm Technol 51:879–886
Negoro H (1978) Inulase from Kluyveromyces fragilis. J Ferm Technol 56:102–107
Negoro H, Kito E (1973) β-fructofuranosidase from Candida kefyr. J Ferm Technol 51:96–102
Nelson N (1944) A photometric adaptation of the Somogyi method for the determination of glucose. J Biol Chem 153:375–380
Rutherford PP, Deacon AC (1972) β-fructofuranosidases from roots of Dandelion (Taraxacum officinale). Biochem J 126:569–573
Snyder HE, Phaff HJ (1960) Studies on a β-fructosidase (Inulinase) produced by Saccharomyces fragilis. Ant Van Leuvoenhoek J Microbiol Serol 26:433–452
Somogyi M (1952) Notes on sugar determination. J Biol Chem 105:19–23
Vandamme EJ, Derycke DG (1983) Microbial Inulinases fermentation process, properties and application. Adv Appl Microb 29:139–176
Workman WE, Day DF (1983) Purification and properties of the β-fructofuranosidase from Kluyveromyces fragilis. FEBS Letters 160:16–20
Zittan L (1981) Enzymatic hydrolysis of Inulin. An alternative way to fructose production. Starch 33:373–377
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ettalibi, M., Baratti, J.C. Purification, properties and comparison of invertase, exoinulinases and endoinulinases of Aspergillus ficuum . Appl Microbiol Biotechnol 26, 13–20 (1987). https://doi.org/10.1007/BF00282143
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00282143