Summary
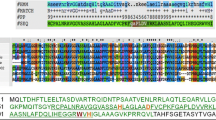
Erwinia chrysanthemi, a phytopathogenic enterobacterium, secretes three proteases (PrtA, PrtB and PrtC) into the extracellular medium. The gene encoding the 50 kDa protease, prtA, was subcloned from a recombinant cosmid carrying a fragment of the E. chrysanthemi B374 chromosome. prtA was shown to be located immediately 3′ to the structural genes for the other two extracellular proteases. The amino acid sequence of PrtA, as predicted from the prtA nucleotide sequence, showed a high level of homology with a family of metalloproteases that are all secreted via a signal peptide-independent pathway, including PrtB and PrtC of E. chrysanthemi B374, PrtC of E. chrysanthemi EC16, PrtSM of Serratia marcescens and AprA of Pseudomonas aeruginosa. PrtA secretion requires the E. chrysanthemi protease secretion factors PrtD, PrtE and PrtF. The secretion signal of PrtA is near to the carboxy-terminal end of the protein, as was previously shown to be the case for PrtB and PrtSM and for Escherichia coli α-hemolysin. The C-termini of these four proteins do not show extensive primary sequence homology, but PrtA, PrtB and PrtSM each have a potential amphipathic α-helix located close to the C-terminus.
Similar content being viewed by others
References
Bouvier J, Pugsley AP, Stragier P (1991) A gene for a new lipoprotein in the dapA-purC interval of the Escherichia coli chromosome. J Bacteriol 173:5523–5531
Chang ACY, Cohen SN (1977) Genome construction between bacterial species in vitro: replication and expression of Staphylococcus plasmid genes in Escherichia coli. Proc Natl Acad Sci USA 71:1030–1034
Colman PM, Jansonius JN, Matthews BW (1972) The structure of thermolysin; an electron density map at 2–3 Å resolution. J Mol Biol 70:701–724
Dahler GS, Barras F, Keen NT (1990) Cloning of genes encoding extracellular metalloproteases from Erwinia chrysanthemi EC16. J Bacteriol 172:5803–5815
Davagnino J, Herrero M, Furlong D, Moreno F, Kolter R (1986) The DNA-replication inhibitor microcin B17 is a forty-three amino-acid protein containing sixty percent glycine. Proteins 1:230–238
Delepelaire P, Wandersman C (1989) Protease secretion by Erwinia chrysanthemi. J Biol Chem 264:9083–9089
Delepelaire P, Wandersman C (1990) Protein secretion in Gram-negative bacteria. J Biol Chem 265:17118–17125
Delepelaire P, Wandersman C (1991) Characterization, localization and transmembrane organization of the three proteins PrtD, PrtE and PrtF necessary for protease secretion by the Gram-negative bacterium Erwinia chrysanthemi. Mol Microbiol 5:2427–2434
Economou A, Hamilton WDO, Johnston AWB, Downie JA (1990) The Rhizobium nodulation gene nodO encodes a Ca2+-binding protein that is exported without N-terminal cleavage and is homologous to haemolysin and related proteins. EMBO J 9:349–354
Eisenberg D, Weiss R, Terwilliger TC (1984) The hydrophobic moment detects periodicity in protein hydrophobicity. Proc Natl Acad Sci USA 81:140–144
Felmlee T, Pellett S, Welch RA (1985) Escherichia coli chromosomal hemolysin is released extracellularly without cleavage of a signal peptide. J Bacteriol 163:88–93
Garnier J, Osguthorpe DJ, Robson B (1978) Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol 120:97–120
Gentschev J, Hess J, Goebel W (1990) Change in the cellular localization of alkaline phosphatase by alteration of its carboxy terminal sequence. Mol Gen Genet 222:211–216
Gilson L, Mahanty HK, Kolter R (1990) Genetic analysis of an MDR-like export system: the secretion of colicin V. EMBO J 9:3875–3884
Glaser P, Ladant D, Sezer O, Pichot F, Ullmann A, Danchin A (1987) The calmodulin-sensitive adenylate cyclase of Bordetella pertussis: cloning and expression in Escherichia coli. Mol Microbiol 2:19–30
Glaser P, Sakamoto H, Belladou J, Ullmann A, Danchin A (1988) Secretion of cyclolysin, the calmodulin-sensitive adenylate cyclase-haemolysin bifunctional protein of Bordetella pertussis. EMBO J 7:3997–4004
Gray L, Baker K, Kenny B, Mackman N, Haigh R, Holland IB (1989) A novel C-terminal signal sequence targets Escherichia coli haemolysin directly to the medium. J Cell Sci Suppl 11:45–47
Guzzo J, Pages JM, Duong F, Lazdunski A, Murgier M (1991a) Pseudomonas aeruginosa alkaline protease: evidence for secretion genes and study of secretion mechanism. J Bacteriol 173:5290–5297
Guzzo J, Duong F, Wandersman C, Murgier M, Lazdunski A (1991b). The secretion genes of Pseudomonas aeruginosa alkaline protease are functionally related to those of Erwinia chrysanthemi proteases and Escherichia coli haemolysin. Mol Microbiol 5:447–453
von Heijne G (1986) Mitochondrial targeting sequence, may form amphiphilic helices. EMBO J 5:1335–1342
Hewlett EL, Gray L, Allietta L, Ehrmann I, Garden YM, Gray MC (1991) Adenylate cyclase toxin from Bordetella pertussis. J Biol Chem 266:17503–17508
Higgins DG, Sharp PM (1988) Clustal: a package for performing multiple sequence alignment on a microcomputer. Gene 73:237–244
Kenny B, Taylor S, Holland B (1992) Identification of individual amino acids required for secretion within the haemolysin (HlyA) C-terminal targeting region. Mol Microbiol 6:1477–1489
Koronakis V, Koronakis E, Hughes C (1989) Isolation and analysis of the C-terminal signal directing export of Escherichia coli hemolysin protein across both bacterial membranes. EMBO J 8:595–605
Kyte J, Doolittle RF (1982) A simple method for displaying the hydropathic character of a protein. J Mol Biol 157:105–132
Létoffé S, Delepelaire P, Wandersman C (1989) Characterization of a protein inhibitor of extracellular proteases produced by Erwinia chrysanthemi. Mol Microbiol 3:79–86
Létoffé S, Delepelaire P, Wandersman C (1990) Protease secretion by Erwinia chrysanthemi: the specific secretion functions are analogous to those of Escherichia coli α-haemolysin. EMBO J 9:1375–1382
Létoffé S, Delepelaire P, Wandersman C (1991) Cloning and expression in Escherichia coli of the Serratia marcescens metalloprotease gene: secretion of the protease from E. coli in the presence of the Erwinia chrysanthemi protease secretion functions. J Bacteriol 173:2160–2166
Lipman DJ, Pearson WR (1985) Rapid and sensitive protein similarity searches. Science 227:1435–1441
Ludwig A, Jarchau T, Benz R, Goebel W (1988) The repeat domain of Escherichia coli haemolysin (HlyA) is responsible for its Ca2+-dependent binding to erythrocytes. Mol Gen Genet214:553–561
Mackman N, Baker K, Gray L, Haig R, Nicaud JM, Holland IB (1987) Release of a chimeric protein into the medium from Escherichia coli using the C-terminal secretion signal of haemolysin. EMBO J 6:2835–2841
Nakahama K, Yoshimura Y, Marumoto R, Kikuchi M (1986) Cloning and sequencing of Serratia protease gene. Nucleic Acids Res 14:5843–5854
Neurath H (1984) Evolution of proteolytic enzymes. Science 224:350–357
Okuda K, Morihara K, Atsumi Y, Takeuchi H, Kawamoto S, Kawasaki H, Suzuki K, Fukushima J (1990) Complete nucleotide sequence of the structural gene for alkaline proteinase from Pseudomonas aeruginosa IFO 3455. Infect Immun 56:4083–4088
Pugsley AP (1988) Protein secretion across the outer membrane of Gram-negative bacteria. In: Das RA, Robbins PW (eds) Protein transfer and organelle biogenesis. Academic Press, pp 607–652
Pugsley AP (1991) Superfamilies of bacterial transport systems with nucleotide binding components. In: Mohan SB, Dow C, Cole JA (eds) Prokaryote structure and function: a new perspective. Society for General Microbiology, Symposium 47, Cambridge University Press, Cambridge, pp 223–248
Roise D, Horvath SJ, Tomich JM, Richards JH, Schatz G (1986) A chemically synthesized pre-sequence of an imported mitochondrial protein can form an amphiphilic helix and perturb natural and artificial phospholipid bilayers. EMBO J 5:1327–1334
Sambrook J, Fritsch EF, Maniatis TE (1989) Molecular cloning: A laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74:5463–5467
Spratt BG, Hedge PJ, Heesen ST, Edelman A, Broome-Smith JK (1986) Kanamycin-resistant vectors that are analogues of plasmids pUC8, pUC9, pEMBL8 and pEMBL9. Gene 41:337–342
Stanley P, Koronakis V, Hugues C (1991) Mutational analysis supports a role for multiple structural features in the C-terminal secretion signal of Escherichia coli haemolysin. Mol Microbiol 5:2391–2403
Tamaki SJ, Gold S, Robeson M., Manulis S, Keen NT (1988) Structure and organization of the peI genes from Erwinia chrysanthemi EC16. J Bacteriol 170:3468–3478
Wandersman C, Andro T, Bertheau Y (1986) Extracellular proteases in Erwinia chrysanthemi. J. Gen Microbiol 132:899–906
Wandersman C, Delepelaire P, Létoffé S, Schwartz M (1987) Characterization of Erwinia chrysanthemi extracellular proteases: cloning and expression of the protease genes in Escherichia coli. J Bacteriol 169:5046–5053
Welch RA (1991) Pore-forming cytolysins of Gram-negative bacteria. Mol Microbiol 5:521–528
Wickner W, Driessen AJM, Hartl FU (1991) The enzymology of protein translocation across the Escherichia coli plasma membrane. Annu Rev Biochem 60:101–124
Author information
Authors and Affiliations
Additional information
Communicated by W. Goebel
Rights and permissions
About this article
Cite this article
Ghigo, JM., Wandersman, C. Cloning, nucleotide sequence and characterization of the gene encoding the Erwinia chrysanthemi B374 PrtA metalloprotease: a third metalloprotease secreted via a C-terminal secretion signal. Molec. Gen. Genet. 236, 135–144 (1992). https://doi.org/10.1007/BF00279652
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00279652