Summary
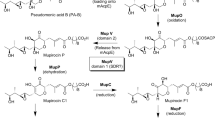
Micromonospora olivasterospora, a fortimicin A (FTM A, astromicin) producer, was found to carry an enzyme that converts FTM A to N-formimidoyl FTM A (FI-FTM A). This enzyme (FI-FTMase) was purified to homogeneity and shown to be a flavin adenine dinucleotide (FAD) enzyme. Tracer experiments proved that the formimidoyl group was derived from C-2 of glycine via oxidation of the amino acid in the presence of FTM A and oxygen. The gene encoding this enzyme, fms 14, was cloned using a 26-mer oligonucleotide probe, designed according to the N-terminal amino acid sequence of purified FI-FTMase, from a cosmid clone pGLM990, which has been shown to contain a cluster of FTM A biosynthetic genes. The nucleotide sequence, and biochemical and genetic analysis revealed that FI-FTMase is composed of four identical subunits of mol. wt. 52000, and contains at least one FAD per subunit. DNA regions homologous to fms14 were found in two other producers of the fortimicin group of antibiotics, Dactylosporangium matsuzakiense ATCC31570 and Micromonospora sp. SF-2098.
Similar content being viewed by others
References
Allain CC, Poon LS, Chan CSG, Richmond W, Fu PC (1974) Enzymatic determination of total serum cholesterol. Clin Chem 20:470–475
Baum EZ, Love SF, Rothstein DM (1988) Temporally regulated tandem promoters in Micromonospora echinospora. J Bacteriol 170:71–77
Baum EZ, Buttner MJ, Lin LS, Rothstein DM (1989) Transcription from the pl promoters of Micromonospora echinospora in the absence of native upstream DNA sequences. J Bacteriol 171:6503–6510
Bibb MJ, Findlay PR, Johnson MW (1984) The relationship between base composition and codon usage in bacterial genes and its use in the simple and reliable identification of protein coding sequence. Gene 30:157–166
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Dairi T, Hasegawa M (1989) Common biosynthetic feature of fortimicin-group antibiotics. J Antibiot 42:934–943
Dairi T, Ohta T, Hashimoto E, Hasegawa M (1992) Organization and nature of fortimicin A (astromicin) biosynthetic genes studied by a cosmid library of Micromonospora olivasterospora. Mol Gen Genet 236:39–48
Deushi T, Iwasaki A, Kamiya K, Mizoguchi T, Nakayama M, Itoh H, Mori T (1979) New aminoglycoside antibiotics, sannamycin. J Antibiot 32:1061–1065
Goldberg SL, Romero JG, Deo YM (1990) Cloning and characterization of the sisomicin-resistance gene from Micromonospora inyoensis. J Antibiot 43:992–999
Grasselli JG (1973) Atlas of spectral data and physical constants for organic compounds. CRC Press Boca Raton
Hanukoglu I, Gutfinger T (1989) cDNA sequence of adrenodoxin reductase. Eur J Biochem 180:479–484
Hasegawa M, Dairi T, Ohta T, Hashimoto E (1991) A novel, highly efficient gene-cloning system for Micromonospora strains. J Bacteriol 173:7004–7011
Hausinger RP, Honek JF, Walsh C (1986) Separation of flavins and flavin analogs by high-performance liquid chromatography. Methods Enzymol 122:199–209
Hopwood DA, Bibb MJ, Chater KF, Kieser T, Bruton CJ, Kieser HM, Lydiate DJ, Smith CP, Ward JM, Schremp H (1985) Gene manipulation of Streptomyces, a laboratory manual. The John Innes Foundation, Norwich, UK
Hotta K, Morioka M, Okami Y (1989) Biosynthetic similarity between Streptomyces tenjimariensis and Micromonospora olivasterospora which produce fortimicin-group antibiotics. J Antibiot 42:745–751
Iwasaki A, Itoh H, Mori T (1979) A new broad-spectrum aminoglycoside antibiotic complex, sporaricin. II. Taxonomic studies on the sporaricin producing strain Saccharopolyspora hirsuta subsp. kobensis nov. subsp. J Antibiot 32:180–186
Kelemen GH, Cundliffe E, Financsek I (1991) Cloning and characterization of gentamicin-resistance genes from Micromonospora purpurea and Micromonospora rosea. Gene 98:53–60
Kieser T (1984) Factors affecting the isolation of cccDNA from Streptomyces lividans and Esherichia coli. Plasmid 12:19–36
Kondo S, Horiuchi Y, Ikeda D, Gomi S, Hotta K, Okami Y, Umezawa H (1982) 2″-N-Forminidoyl-istamycin A and B produced by Streptomyces tenjimariensis. J Antibiot 35:1104–1106
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning, a laboratory mannual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Nara T, Yamamoto M, Kawamoto I, Takayama K, Okachi R, Takasawa S, Sato T, Sato S (1977) Fortimicins A and B, new aminoglycoside antibiotics. I. Producing organisms, fermentation and biological properties of fortimicins. J Antibiot 30:533–540
Ohba K, Shomura T, Tsuruoka T, Kojima M, Inouye S, Itoh T (1981) Production of antibiotics SF-2050. Patent JP18600 Feb. 21, 1981
Ohta T, Nagano E, Dairi T, Hasegawa M (1988) In: 7th International Symposium on Biology of Actinomycetes, Japan, May 22–26, P3–29
Okami Y, Hotta K, Yoshida M, Ikeda D, Kondo S, Umezawa H (1979) New aminoglycoside antibiotics, istamycin A and B. J Antibiot 32:964–966
Shomura T, Kojima M, Yoshida J, Ito M, Asano S, Totsugawa K, Niwa T, Inouye S, Ito T, Niida T (1980) Studies on a new aminoglycoside antibiotic, dactimicin. I. Producing organism and fermentation. J Antibiot 33:924–930
Thompson J, Skeggs PA, Cundliffe E (1985) Methylation of 16S ribosomal RNA and resistance to the aminoglycoside antibiotics gentamicin and kanamycin determined by DNA from the gentamicin producer, Micromonospora purpurea. Mol Gen Genet 201:168–173
Umezawa H, Gomi S, Yamagishi Y, Obata T, Ikeda T, Hamada M, Kondo S (1987) 2″-N-formimidoylsporaricin A produced by Saccharopolyspora hirsuta subsp. kobensis. J Antibiot 40:91–93
Van Hoof F, Heyndrickx (1974) Thin layer chromatographic-spectrophotofluorometric analysis of amphetamine and amphetamine analog after reaction with 4-chloro-7-nitrobenzo-2, 1, 3-oxadiazole. Anal Chem 46:286–288
Vieira J, Messing J (1987) Production of single-stranded plasmid DNA. Methods Enzymol 153:3–11
Whilby LG (1953) A new method for preparing flavin adenine dinucleotide. Biochem J 54:437–442
Yanisch-Perron C, Vieira J, Messing J (1985) Improved M13 phage cloning vectors and host strains: nucleotide sequence of the M13 mp18 and pUC19 vectors. Gene 33:103–119
Author information
Authors and Affiliations
Additional information
Communicated by H. Hennecke
Rights and permissions
About this article
Cite this article
Dairi, T., Yamaguchi, K. & Hasegawa, M. N-Formimidoyl fortimicin A synthase, a unique oxidase involved in fortimicin A biosynthesis: purification, characterization and gene cloning. Molec. Gen. Genet. 236, 49–59 (1992). https://doi.org/10.1007/BF00279642
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00279642