Abstract
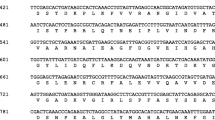
A Nicotiana tabacum thioredoxin h gene (EMBL Accession No. Z11803) encoding a new thioredoxin (called h2) was isolated using thioredoxin h1 cDNA (X58527), and represents the first thioredoxin h gene isolated from a higher plant. It encodes a polypeptide of 118 amino acids with the conserved thioredoxin active site Trp-Cys-Gly-Pro-Cys. This gene comprises two introns which have lengths of 1071 and 147 by respectively, and three exons which encode peptides of 29, 41 and 48 amino acids, respectively. This thioredoxin h shows 66% identity with the amino acid sequence of thioredoxin h1 (X58527) and only around 35% with the choroplastic thioredoxins. The two thioredoxins, h1 and h2, do not have any signal peptides and are most probably cytoplasmic. Using the 3′ regions of the mRNAs, two probes specific for thioredoxins h1 and h2 have been prepared. Southern blot analysis shows that thioredoxin sequences are present in only two genomic EcoRI fragments: a 3.3 kb fragment encodes h1 and a 4.5 kb fragment encodes h2. Analysis of the ancestors of the allotetraploid N. tabacum shows that thioredoxin h2 is present in N. sylvestris and N. tomentosiformis but that thioredoxin h1 is absent from both putative ancestors. Thus, the thioredoxin h1 gene has probably been recently introduced in to N. tabacum as a gene of agronomic importance, or linked to such genes. Northern blot analysis shows that both genes are expressed in N. tabacum, mostly in organs or tissues that contain growing cells. Thioredoxin h1 is always expressed at a lower level than h2 in tobacco plants. In contrast, the thioredoxin hl gene is abundantly expressed in freshly isolated protoplasts, while h2 mRNAs are not detectable.
Similar content being viewed by others
References
Axelos M, Barbet C, Liboz T, le Van Thai A, Curie C, Lescure B (1989) The gene family encoding the Arabidopsis thaliana translation elongation factor EF-1α: molecular cloning, characterization and expression. Mol Gen Genet 219:106–112
Bolle PA, Gilliquet V, Berben G, Dumont J, Hilger F (1992) Yeast sequencing reports: The complete sequence of K3B, a 7.9 kb fragment between PGK1 on chromosome III, reveals the presence of seven open reading frames. Yeast 8:205–213
Brown JWS (1986) A catalogue of splice junction and putative branch point sequences from plant introns. Nucleic Acids Res 14:9549–9559
Buchanan BB (1980) Role of light in chloroplast enzymes. Annu Rev Plant Physiol 31:341–374
Chaboute ME, Chaubet N, Philipps G, Gigot C (1987) Genomic organization and nucleotide sequences of two histone H3 and two histones H4 genes of Arabidopsis thaliana. Plant Mol Biol 8:179–191
Chen NY, Zhang JJ, Paulus H (1989) Nucleotide sequence of the overlapping genes for the subunits of Bacillus subtilis aspartokinase II and their control regions. J Biol Chem 262:8787–8798
Curie C, Liboz T, Barbet C, Gander E, Medale C, Axelos M, Lescure B (1991) Cis and trans-acting elements involved in the activation of Arabidopsis thaliana A1 gene encoding the translation elongation factor EF-1α. Nucleic Acids Res 19:1305–1310
Curie C, Liboz T, Montana MH, Ronan D, Axelos M, Lescure B (1992) The activation process of Arabidopsis thaliana A1 gene encoding the translation elongation factor EF-1α is conserved among angiosperms. Plant Mol Biol 18:1083–1090
Cséke C, Buchanan BB (1986) Regulation of the formation and utilization of photosynthate in leaves. Biochem Biophys Acta 853:43–63
Decottignies P, Schmitter JM, Jacquot JP, Dutka S, Picaud A, Gadal P (1990) Purification, characterization, and complete amino acid sequence of a thioredoxin from a green alga, Chlamydomonas reinhardtii. Arch Biochem Biophys 280:112–121
Decottignies P, Schmitter JM, Dutka S, Jacquot JP, Miginiac-Maslow M (1991) Characterization and primary structure of a second thioredoxin from the green alga, Chlamydomonas reinhardtii. Eur J Biochem 198:505–512
Delcasso-Tremousaygue F, Pannabieres F, Grellet F, Ananiev E, Delseny M (1988) Structural and transcriptional characterization of the external spacer of a ribosomal RNA nuclear gene from a higher plant. Eur J Biochem 172:767–776
Droux M, Jacquot JP, Miginiac-Maslow M, Gadal P, Huct JC, Crawford NA, Yee BC, Buchanan BB (1987) Ferredoxin-thioredoxin reductase: an iron-sulfur enzyme linking light to enzyme regulation in oxygenic photosynthesis. Purification and properties of the enzyme from C3, C4 and cyanobacterial species. Arch Biochem Biophys 252:426–439
Dubbendorff JW, Studier FW (1991) Controlling basal expression in an inducible T7 expression system by blocking the target T7 promotor with 1ac repressor. J Mol Biol 219:45–59
Florencio FJ, Yee BC, Johnson TC, Buchanan BB (1988) An NADP/thioredoxin system in leaves: purification and characterization of NADP-thioredoxin reductase and thioredoxin h from spinach. Arch Biochem Biophys 266:496–507
Freedman RB, Hawkins HC, Murant SJ, Reid L (1988) Protein disulphide-isomerase: a homologue of thioredoxin implicated in the biosynthesis of secretory proteins. Biochem Soc Trans 16:96–99
Gan ZR (1991) Yeast thioredoxin genes. J Biol Chem 266:1692–1696
Gleason FK, Holmgren A (1988) Thioredoxin and related proteins in procaryotes. FEMS Microbiol Rev 54:271–298
Gleason FK, Whittaker MM, Holmgren A, Jörnvall H (1985) The primary structure of thioredoxin from the filamentous cyanobacterium Anabaena sp. 7119. J Biol Chem 260:9567–9573
Grosset J, Marty I, Chartier Y, Meyer Y (1990) mRNAs newly synthesized by tobacco mesophyll protoplasts are wound inducible. Plant Mol Biol 15:485–496
Higgins DG, Bleasby AJ, Fuchs R (1992) ClustalV: Improved software for multiple sequence alignment. Comp Appl Biol Sci (in press)
Holmgren A (1989) Thioredoxin and glutaredoxin systems. J Biol Chem 264:13963–13966
Huppe HC, de Lamotte-Guéry F, Jacquot JP, Buchanan BB (1990) The ferredoxin-thioredoxin system of a green alga, Chlamydomonas reinhardtii. Planta 180:341–351
Jacquot JP (1984) Post translational modification of proteins in higher plant chloroplasts: enzyme regulation by thiol disulfite interchange. Physiol Veg 22:487–507
Jacquot JP, Buchanan BB, Martin F, Vidal J (1981) Enzyme regulation in C4 photosynthesis. Purification and properties of thioredoxin-linked NADP-malate dehydrogenase from corn leaves. Plant Physiol 68:300–304
Jouanneau JP, Tandeau de Marsac N (1973) Stepwise effects of cytokinin activity and DNA synthesis upon mitotic cycle events in partially synchronised tobacco cells. Exp Cell Res 77:167–174
Johnson RS, Mathews WR, Biemann K, Hopper S (1988) Amino acid sequence of thioredoxin isolated from rabbit bone marrow determined by tandem mass spectrometry. J Biol Chem 263:9589–9597
Johnson TC, Cao RQ, Kung JE, Buchanan BB (1987a) Thioredoxin and NADP-thioredoxin reductase from cultured carrot cells. Planta 171:321–331
Johnson TC, Wada K, Buchanan BB, Holmgren A (1987b) Reduction of purothionin by the wheat seed thioredoxin system. Plant Physiol 85:446–451
Jones SW, Luk KC (1988) Isolation of a chicken thioredoxin cDNA clone. J Biol Chem 263:9607–9611
Joshi CP (1987) Putative polyadenylation signals in nuclear genes of higher plants: a compilation analysis. Nucleic Acids Res 15:9627–9640
Kamo M, Tsugita A, Wiessner C, Wedel N, Bartling D, Herrmann RG, Aguilar F, Gardet-Salvi L, Schürmann P (1989) Primary structure of spinach chloroplast thioredoxin F: protein sequencing and analysis of complete cDNA clones for spinach chloroplast. Eur J Biochem 154:197–203
Kobrehel K, Yee BC, Buchanan BB (1991) Role of the NADP thioredoxin system in the reduction of alpha-amylase and trypsin-inhibitor proteins. J Biol Chem 266:6135–6140
Lundström J, Holmgren A (1990) Protein disulfide-isomerase is a substrate for thioredoxin reductase and has thioredoxin-like activity. J Biol Chem 265:9114–9120
Maeda K, Tsugita A, Dalzoppo D, Vilbois F, Schürmann (1986) Further characterization and amino acid sequence of m-type thioredoxins from spinach choroplasts. Eur J Biochem 154:197–203
Marck C (1988) Strider 1-1. Nucleic Acids Res 16:1829–1836
Marcus F, Chamberlain SH, Chu C, Masiarz FR, Shin S, Yee BC, Buchanan BB (1991) Plant thioredoxin-H-an animal-like thioredoxin occurring in multiple cell compartments. Arch Biochem Biophys 287:195–198
Marty I, Meyer Y (1991) Nucleotide sequence of a cDNA encoding a tobacco thioredoxin. Plant Mol Biol 17:143–147
Meinkoth J, Wahl G (1984) Hybridisation of nucleic acid immobilized on solid support. Anal Biochem 138:267–284
Meyer Y, Abel WO (1975) Importance of the wall for cell division and in the activity of the cytoplasm in cultured tobacco protoplasts. Planta 123:33–40
Modrich P, Richardson CC (1976) Bacteriophage T7 deoxyribonucleic acid replication in vitro. A protein of Escherichia coli required for bacteriophage T7 DNA polymerase activity. J Biol Chem 250:5508–5514
Muller EGD (1991) Thioredoxin deficiency in yeast prolongs S phase and shortens the G 1 interval of the cell cycle. J Biol Chem 266:9194–9202
Muller EGD, Buchanan BB (1989) Thioredoxin is essential for photosynthetic growth. The thioredoxin gene of Anacystis nidulans. J Biol Chem 264:4008–4014
Pearson WR (1990) Rapid and sensitive comparison with FASTAP and FASTA. Methods Enzymol 183:63–93
Reichard P (1988) Interactions between deoxyribonucleotide and DNA synthesis. Annu Rev Biochem 57:349–374
Rivera-Madrid R, Marinho P, Brugidou C, Chartier Y, Meyer Y (1992) Nucleotide sequence of a cDNA clone encoding an Arabidopsis thaliana thioredoxin h. Plant Physiol, in press
Rogers JH (1990) The role of introns in evolution. FEBS Lett 268:339–343
Russel M, Model P (1985) Thioredoxin is required for filamentous phage assembly. Proc Natl Acad Sci USA 82:29–33
Sanger F, Nicklens S, Coulson AR (1977) DNA sequencing with chain terminating inhibitors. Proc Natl Acad Sci USA 74:5463–5467
Sambrook J, Fritsch EF, Maniatis T (1990) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY
Sauer J, Friedländer K, Gräm-Wicke U (1990) Primary structure, genomic organization and heterologous expression of a glucose transporter from Arabidopsis thaliana. EMBO J 9:3045–3050
Tagaya Y, Maeda Y, Mitsui A, Kondo N, Matsui H, Hamuro J, Brown N, Arai K, Yokota T, Wakasugi H, Yodoi J (1989) ALT-derived factor (Ado, an I1-2 receptor Tac inducer homologous to thioredoxin — possible involvement of dithiol-reduction in the I1-2 receptor induction. EMBO J 8:757–764
flaTasanen K, Parkkonen T, Chow LT, Kivirikko KI, Pihlajaniemi T (1988) Characterization of the human gene for a polypeptide that acts both as the beta subunit of prolyl 4-hydroxylase and as protein disulfide isomerase. J Biol Chem 263:16218–16224
Tonissen KF, Wells JRE (1991) Isolation and characterization of human thioredoxin encoding genes. Gene 102:221–228
Tonissen KF, Robin AJ, Wells JRE (1989) Nucleotide sequence of a cDNA encoding rat thioredoxin. Nucleic Acids Res 17:3973
Tsang MLS, Schiff JA (1976) Sulfate-reducing pathway in Escherichia coli involving bound intermediates. J Bacteriol 125:923–933
Van Langendonckt A, Vanden Driessche (1992) Isolation and characterization of different forms of thioredoxins from the green alga Acetabularia mediterranea: identification of an NADP/thioredoxin system in the extrachloroplastic fraction. Arch Biochem Biophys 292:156–164
Vaucheret H, Vincentz M, Kronenberger J, Caboche M, Rouzé P (1989) Molecular cloning and characterisation of the two homologous genes coding for nitrate reductase in tobacco. Mol Gen Genet 216:10–5
Vogt K, Follmann H (1986) Characterization of three different thioredoxins in wheat. Biochim Biophys Acta 873:415–418
Wedel N, Clausmeyer S, Herrmann RG, Gardet-Salvi L, Schür-mann P (1992) Nucleotide sequence of cDNAs encoding the entire precursor polypeptide for thioredoxin m from spinach chloroplasts. Plant Mol Biol 18:527–533
Wetterauer B, Jacquot JB, Veron M (1992) Thioredoxin from Dictyostelium discoideum are a developmentally regulated multigene family. J Biol Chem, in press
Wollman EE, d'Auriol L, Rimsky L, Shaw A, Jacquot JP, Wingfield P, Graber P, Dessarps F, Robin P, Galibert F, Bertoglio J, Fradelizi D (1988) Cloning and expression of a cDNA for a human thioredoxin. J Biol Chem 263:15506–15512
Author information
Authors and Affiliations
Additional information
Communicated by A. Kondorosi
Rights and permissions
About this article
Cite this article
Brugidou, C., Marty, I., Chartier, Y. et al. The Nicotiana tabacum genome encodes two cytoplasmic thioredoxin genes which are differently expressed. Molec. Gen. Genet. 238, 285–293 (1993). https://doi.org/10.1007/BF00279557
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00279557