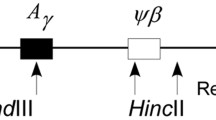
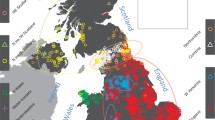
Summary
DNA analysis of the α- and β-globin gene clusters has revealed substantial variability between individuals and populations. As well as restriction enzyme site and length polymorphisms, variation in gene copy number and type is observed. Because of this extensive polymorphism DNA analysis offers a highly informative method of studying genetic affinities between human populations. Haplotypes, consisting of a set of restriction enzyme polymorphisms distributed along the cluster, have been developed for both loci. Analysis of the molecular basis of numerous β-thalassaemia alleles has revealed, in general, different sets of mutations in different populations, indicating that these postdate the racial divergence. Recent microepidemiological studies on the distribution of α-thalassaemia support the hypothesis that this condition, like the {ie16-1}, has been selected because it confers protection against malaria. Population-specific DNA polymorphisms at these and other loci promise to be of considerable value to genetic anthropology.
Similar content being viewed by others
References
Antonarakis SE, Boehm CD, Giardina PJV, Kazazian HH Jr (1982a) Nonrandom association of polymorphic restriction sites in the β-globin gene cluster. Proc Natl Acad Sci USA 79:137–141
Antonarakis SE, Orkin SH, Kazazian HH Jr, Goff SC, Boehm CD, Waber PG, Sexton JP, Ostrer H, Fairbanks VF, Charkravarti A (1982) Evidence for multiple origins of the 22-1 gene in Southeast Asia. Proc Natl Acad Sci USA 79:6608–6611
Antonarakis SE, Boehm CD, Serjeant GR, Theisen CE, Dover GJ, Kazazian HH Jr (1984a) Origin of the 22-2 globin gene in Blacks: the contribution of recurrent mutation or gene conversion or both. Proc Natl Acad Sci USA 81:853–856
Antonarakis SE, Orkin SH, Cheng T-c, Scott AF, Sexton JF, Trusco S., Charache S, Kazazian HH Jr (1984b) β-thalassemia in American Blacks: novel mutations in the TATA box and IVS-2 acceptor splice site. Proc Natl Acad Sci USA 81:1154–1158
Antonarakis SE, Kazazian HH, Orkin SH (1985) DNA polymorphism and molecular pathology of the human globin gene clusters. Hum Genet 69:1–14
Baltimore D (1981) Gene conversion: some implications for immunoglobulin genes. Cell 24:592–594
Black RH (1955) The geographical distribution of malaria in the south-west Pacific. Aust Geographer 6:32–36
Boehm CD, Dowling CE, Antonarakis SE, Honig GR, Kazazian HH (1985) Evidence supporting a single origin of the 22-3 gene in Blacks. Am J Hum Genet 37:771–777
Cavalli-Sforza LL, Bodmer WG (1971) The genetics of human populations. Freeman, San Francisco
Cavalli-Sforza LL (1985) Current knowledge of the human genome and outlook for the future (abstr). Paris meeting (in press)
Chakravarti A, Buetow KH, Antonarakis SE, Waber PG, Boehm CD, Kazazian HH Jr (1984) Nonuniform recombination within the human β-globin gene cluster. Am J Hum Genet 36:1239–1258
Chehab FF, Honig GR, Kan YW (1986) Spontaneous mutation in β-thalassaemia producing the same nucleotide substitution as that in a common hereditary form. Lancet I:3–5
Cheng T-c, Orkin SH, Antonarakis SE, Potter MJ, Sexton JP, Markham AF, Giardina PVJ, Li A, Kazazian HH Jr (1984) β-thalassemia in Chinese: use of in vivo RNA analysis and oligonucleotide hybridization in systematic characterization of molecular defects. Proc Natl Acad Sci USA 81:2821–2825
Collins FS, Weissman SM (1984) The molecular genetics of human hemoglobin. Prog Nucleic Acid Res Mol Biol 3:315–462
Collins FS, Boehm CD, Waber PG, Stoeckert CJ, Weissman SM, Forget BG, Kazazian HH (1984) Concordance of a point mutation 5′ to the GJ globin gene with 22-4 hereditary persistence of fetal hemoglobin in the black population. Blood 64:1292–1296
Cooper DN, Schmidtke J (1984) DNA restriction fragment length polymorphisms and heterozygosity in the human genome. Hum Genet 66:1–17
Deisseroth A, Nienhuis A, Turner P, Velez R, Anderson WF, Ruddle F, Lawrence J, Creagan R, Kucherlapati R (1977) Localization of the human α-globin structural gene to chromosome 16 in somatic cell hybrids by molecular hybridization asay. Cell 12:205–218
Deisseroth A, Nienhuis A, Lawrence J, Giles R, Turner P, Ruddle FH (1978) Chromosomal localization of human β-globin gene on human chromosome 11 in somatic cell hybrids. Proc Natl Acad Sci USA 75:1456–1460
Flint J, Hill AVS, Bowden DK, Oppenheimer SJ, Sill PR, Serjeartson SW, Banakoiri J, Bhatia K, Alpers MP, Boyce AJ, Weatherall OJ, Clegg JB (1986) High frequencies of α thalassaemia are the result of natural selection by malaria. Nature 321:744–750
Friedman MJ (1978) Erythrocytic mechanisms of sickle resistance to malaria. Proc Natl Acad Sci USA 75:1994–1997
Gerhard DS, Kidd KK, Kidd JR, Egeland JA, Housman DE (1984) Identification of a recent recombination event within the human β-globin gene cluster. Proc Natl Acad Sci USA 81:7875–7879
Giles RE, Blanc H, Cann HM, Wallace DC (1980) Maternal inheritance of human mitochondrial DNA. Proc Natl Acad Sci USA 77:6715–6719
Gilman JB, Huisman THJ (1985) DNA sequence variation associated with elevated fetal 22-5 globin production. Blood 66:783–787
Goodbourn SEY, Higgs DR, Clegg JB, Weatherall DJ (1983) Molecular basis of length polymorphism in the human α-globin gene complex. Proc Natl Acad Sci USA 80:5022–5026
Goodbourn SEY, Higgs DR, Clegg JB, Weatherall DJ (1984) Allelic variation and linkage properties of a highly polymorphic restriction fragment in humans. Mol Biol Med 2:223–238
Goosens M, Dozy AN, Embury SH, Zachariades Z, Hadjimias MG, Stamatoyannopoulos G, Kan YW (1980) Triplicated α-globin loci in humans. Proc Natl Acad Sci USA 77:518–521
Haldane JBS (1949) The rate of mutation of human genes. Proceedings of 8th International Congress on Genetics, pp 267–273
Harano K, Harano T, Kutlar F, Huisman THJ (1985) γ-globin triplication and quadruplication in Japanese newborns. FEBS Lett 190:45–49
Hardison RC, Sanvada I, Cheng J-F, Shen C-KJ, Schmid CW (1986) A previously undetected pseudogene in the human alpha globin gene cluster. Nucleic Acids Res 14:1903–1911
Harris S, Barrie PA, Weiss ML, Jeffreys AJ (1984) The primate ψβ1 gene. J Mol Biol 180:785–801
Heyerdahl T (1950) Kon-Tiki: across the Pacific by raft. McNally, New York
Higgs DR, Old JM, Pressley L, Clegg JB, Weatherall DJ (1980) A novel globin gene arrangement in man. Nature 284:632–635
Higgs DR, Goodbourn SEY, Wainscoat JS, Clegg JB, Weatherall DJ (1981) Highly variable regions of DNA flank the human α-globin genes. Nucleic Acids Res 9:4213–4224
Higgs DR, Weatherall DJ (1983) Alpha-thalassaemia. Curr Top Hematol 4:37–97
Higgs DR, Wainscoat JS, Flint J, Hill AVS, Thein SL, Nicholls RD, Teal H, Ayyub H, Peto TEA, Jarman AP, Clegg JB, Weatherall DJ (1986) Analysis of the human α-globin gene cluster reveals a highly informative genetic locus. Proc Natl Acad Sci USA (in press)
Hill AVS (1986) The population genetics of α thalassaemia and the malaria hypothesis. Cold Spring Harbor Symp Quant Biol, vol 51 (in press)
Hill AVS, Bowden DK, Trent RJ, Higgs DR, Oppenheimer SJ, Thein SL, Mickleson KNP, Weatherall DJ, Clegg JB (1985a) Melanesians and Polynesians share a unique α-thalassaemia mutation. Am J Hum Genet 37:571–580
Hill AVS, Nicholls RD, Thein SL, Higgs DR (1985b) Recombination within the human embryonic χ-globin locus: a common χ-χ chromosome produced by gene conversion of the ψχ gene. Cell 42:809–819
Hill AVS, Bowden DK, Weatherall DJ, Clegg JB (1986) Chromosomes with one, two, three and four fetal globin genes: molecular and hematological analysis. Blood (in press)
Huisman THJ, Kutlar F, Nakatsuji T, Bruce Tagoe A, Kilinc Y, Canchi MN, Romero Garcia C (1985) The frequency of the γ chain variant 22-6 in different populations, and its use in evaluating γ gene expression in association with thalassaemia. Hum Genet 71:127–133
Ifediba TC, Stern A, Ibrahim A, Rieder RF (1985) Plasmodium falciparum in vitro: diminished growth in hemoglobin H disease erythrocytes. Blood 65:452–455
Jarman AP, Nicholls DR, Weatherall DJ, Clegg JB, Higgs DR (1986) Molecular characterisation of a hypervariable region downstream of the human α-blobin gene cluster. EMBO J (in press)
Jeffereys AJ (1979) DNA sequence variants in the 22-7, 22-8, δ and β globin genes of man. Cell 18:1–10
Jeffreys AJ (1982) Evolution of globin genes In: Genome evolution. Academic Press, New York London, pp 157–176
Johnson JM, Wallace DC, Ferris SD, Ratlazzi MC, Cavalli-Sforza LL (1983) Radiation of human mitochondrial DNA types analyzed by restriction endonuclease cleavage patterns. J Mol Evol 19:255–271
Jones JS, Rouhani S (1986) Human vvolution: how small was the bottleneck. Nature 319:449–450
Jorde LB (1985) Human genetic distance studies: present status and future prospects. Annu Rev Anthropol 14:343–373
Kan YW, Dozy AM (1978) Polymorphism of DNA sequence adjacent to the human β-globin structural gene: relationship to sickle mutation. Proc Natl Acad Sci USA 75:5631–5635
Kan YW, Dozy AM (1980) Evolution of the hemoglobin S and C genes in world populations. Science 209:492–493
Kazazian HH Jr, Orkin SH, Antonarakis SE, Sexton JP, Boehm CD, Goff SC, Waber PG (1984a) Molecular characterization of seven β-thalassaemia mutations in Asian Indians. EMBO J 3:593–596
Kazazian HH Jr, Orkin SH, Markham AF, Chapman CR, Youssoufian HA, Waber PG (1984b) Quantitation of the close association between DNA haplotypes and specific β-thalassaemia mutations in Mediterraneans. Nature 300:152–154
Kazazian HH Jr, Waber PG, Boehm CD, Lee JI, Antonarakis SE, Fairbank VF (1984c) Hemoglobin E in Europeans: further evidence for multiple origins of the 23-1 gene. Am J Hum Genet 36:212–217
Klein HL, Petes TD (1981) Intrachromosomal gene conversion in yeast. Nature 289:144–148
Koop BF, Goodman M, Xa P, Chan K, Slightom JL (1986) Primate η-globin DNA sequences and man's place among the great apes. Nature 319:234–237
Kulozik AE, Wainscoat JS, Scrjeant GR, Al-Awamy B, Essan F, Falusi Y, Hague SK, Hilali AM, Kate S, Ranasinghe WACP. Weatherall DJ (1986) Geographical survey of {ie23-2} globin gene haplotypes: evidence for an independent Asian origin of the sicklecell mutation. Am J Hum Genet (in press)
Lauer J, Shen C-KJ, Maniatis T (1980) The chromosomal arrangement of human α-like globin genes. Sequence homology and α-globin gene deletions. Cell 20:119–130
Liebhaber SA, Rappaport EF, Cash FE, Ballas SK, Schwartz E, Surrey S (1984) Hemoglobin I mutation encoded at both alpha-globin loci on the same chromosome: concerted evolution in the human genome. Science 226:1449–1451
Livingstone FB (1983) The malaria hypothesis. In: Bowman J (ed) The distribution and evolution of the hemoglobin and globin gene loci. Elsevier, New York, pp 15–44
Maeda N, Bliska JB, Smithies O (1983) Recombination and balanced chromosome polymorphism suggested by DNA sequences 5′ to the human δ-globin gene. Proc Natl Acad Sci USA 80:5012–5016
Marks J, Show J-P, Shen C-KJ (1983) Sequence organisation and complexity of the primate δ1 globin gene, a novel α-globin like gene. Nature (in press)
Molineaux L, Gramiccia G (1980) The Garki project: research on the epidemiology and control of malaria in the Sudan Savanna of West Africa. WHO, Geneva, pp 213–229
Motulsky AG (1964) Hereditary red cell traits and malaria. Am J Trop Hyg 13:147–156
Nagel RL (1984) The origin of the hemoglobin S gene: clinical, genetic, and anthropological consequences. The Einstein Quarterly J Med 2:53–62
Nei M, Roychoudbury AK (1982) Genetic relationship and evolution of human races. In: Krecht MK, Wallace B, Prance GT (eds) Evolutionary biology, vol 14. Plenum Press, New York, pp 1–59
Old JM, Heath C, Fitches A, Thein SL, Jeffreys AJ, Petrou M, Modell B, Weatherall DJ (1986) Meiotic recombination between two polymorphic restriction sites within the β-globin gene cluster. J Med Genet 23:14–18
Oppenheimer SJ, Higgs DR, Weatherall DJ, Barker J, Spark RA (1984) α-thalassaemia in Papua New Guinea. Lancet I:424–426
Orkin SH, Kazazian HH Jr, Antonarakis SE, Goff SC, Boehm CD, Sexton JP, Waber PG, Giardina PVJ (1982) Linkage of β-thalassaemia mutations and β-globin gene polymorphisms with DNA polymorphic in the human β-globin gene cluster. Nature 296:627–631
Pagnier J, Mears JG, Dunda-Belkhodja O, Schaefer-Rego KE, Beldjord C, Nagel RL, Labie D (1984) Evidence for the multicentric origin of the sickle cell hemoglobin gene in Africa. Proc Natl Acad Sci USA 81:1771–1773
Pasvol G, Weatherall DJ, Wilson RJM (1978) Cellular mechanism for the protective effect of haemoglobin S against P. falciparum malaria. Nature 274:701–703
Powars PA, Smithies O (1986) Short gene conversions in the human fetal globin gene region: a by-product of chromosomal pairing during meiosis? Genetics 112:343–358
Proudfoot NJ, Maniatis T (1980) The structure of a human α-globin pseudogene and its relationship to α-globin gene duplication. Cell 21:537–544
Proudfoot NJ, Gil A, Maniatis T (1982) The structure of the human zeta-globin gene and a closely linked, nearly identical pseudogene. Cell 31:553–563
Reeders ST, Breuning MH, Davies KE, Nicholls RD, Jarman AP, Higgs DR, Pearson PL, Weatherall DJ (1985) A highly polymorphic DNA marker linked to adult polycystic kidney disease on chromosome 16. Nature 317:542–544
Rosatelli C, Falchi AM, Tuveri T, Scalas MT, DiTucci A, Monni G, Cao A (1985) Prenatal diagnosis of beta-thalassaemia with the synthetic-oligomer technique. Lancet I:241–243
Scott AF, Health P, Trusko S, Boyer SH, Prass W, Goodman M, Czelusniak J, Chang L-YE, Slightom JL (1984) The sequence of the gorilla fetal globin genes: evidence for multiple gene conversions in human evolution. Mol Biol Evol 1:371–389
Shimasaki S, Iuchi I (1986) Diversity of human γ-globin gene loci including a quadruplicated arrangement. Blood 67:784–788
Slightom JL, Blechl AE, Smithies O (1980) Human fetal 23-3 and 23-4 globin genes: complete nucleotide sequences suggest that DNA can be exchanged between these duplicated genes. Cell 21:627–638
Sukumaran PK, Wakatsuji T, Gardiner MB, Gilman JG, Huisman THJ (1983) Gamma thalassaemia resulting from deletion of a γ-globin gene. Nucleic Acids Res 11:4635–4643
Thein SL, Old JM, Wainscoat JS, Petrou M, Modell B, Weatherall DJ (1984) Population and genetic studies suggest a single origin for the Indian deletion 23-5 thalassaemia. Br J Hacmatol 57:271–278
Thein SL, Wainscoat JS, Old JM, Wallace RB, Weatherall DJ (1985) The AvaII ψβ polymorphism is linked to the common Mediterranean 23-6 thalassaemia mutation. Br J Haematol 61:747–748
Trent RJ, Bowden DK, Old JM, Wainscoat JS, Clegg JB, Weatherall DJ (1981) A novel rearrangement of the human β-like globin gene cluster. Nucleic Acids Res 9:6723–6733
Vogel F, Motulsky AG (1982) Human Genetics: problems and approaches. Springer, New York Berlin Heidelberg, pp 399–404
Wainscoat JS, Bell JI, Thein SL, Higgs DR, Serjeant GR, Peto TEA, Weatherall DJ (1983) Multiple origins of the sickle mutation: evidence from 23-7 globin gene cluster polymorphisms. Mol Biol Med 1:191–197
Wainscoat JS, Old JM, Thein SL, Weatherall DJ (1984) A new DNA polymorphism for prenatal diagnosis of β-thalassaemia in Mediterranean populations. Lancet II:1299–1301
Wainscoat JS, Thein SL, Higgs DR, Bell JI, Weatherall DJ, Al-Awamy B, Serjeant GR (1985) A genetic marker for elevated levels of haemoglobin F in sickle cell disease? Br J Haematol 60:261–268
Wainscoat JS, Hill AVS, Boyce AL, Flint J, Hernandez M, Thein SL, Old JM, Lynch JR, Falusi AG, Weatherall DJ, Clegg JB (1986) Evolutionary relationships of human populations from an analysis of nuclear DNA polymorphisms. Nature 319:491–493
Wainscoat JS, Kulozik AE, Ramsay M, Falusi AG, Weatherall DJ (1986) A Taq-1 γ-globin gene polymorphism: an African specific marker. Hum Genet (in press)
Willcox M (1982) Beta thalassaemia and the malaria hypothesis. A study in Liberia. M.D. Thesis, University of Stockholm
Wilson AC, Carlson SS, Whie TJ (1977) Biochemical evolution. Annu Rev Biochem 46:573–639
Winichagoon P, Higgs DR, Goodbourn SEY, Lamb J, Clegg JB, Weatherall DJ (1982) Multiple arrangements of the human embryonic zeta globin genes. Nucleic Acids Res 10:5853–5868
Winichagoon P, Higgs DR, Goodbourn SEY, Clegg JB, Weatherall DJ, Wasi P (1984) The molecular basis of α-thalassaemia in Thailand. EMBO J 3:1813–1818
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hill, A.V.S., Wainscoat, J.S. The evolution of the α- and β-globin gene clusters in human populations. Hum Genet 74, 16–23 (1986). https://doi.org/10.1007/BF00278779
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00278779