Abstract
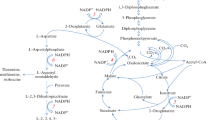
In Nocardia sp. 239 d-phenylalanine is converted into l-phenylalanine by an inducible amino acid racemase. The further catabolism of this amino acid involves an NAD-dependent l-phenylalanine dehydrogenase. This enzyme was detected only in cells grown on l- or d-phenylalanine and in batch cultures highest activities were obtained at relatively low amino acid concentrations in the medium. The presence of additional carbon- or nitrogen sources invariably resulted in decreased enzyme levels. From experiments with phenylalanine-limited continuous cultures it appeared that the rate of synthesis of the enzyme increased with increasing growth rates. The regulation of phenylalanine dehydrogenase synthesis was studied in more detail during growth of the organism on mixtures of methanol and l-phenylalanine. Highest rates of l-phenylalanine dehydrogenase production were observed with increasing ratios of l-phenylalanine/methanol in the feed of chemostat cultures. Characteristic properties of the enzyme were investigated following its (partial) purification from l- and d-phenylalanine-grown cells. This resulted in the isolation of enzymes with identical properties. The native enzyme had a molecular weight of 42 000 and consisted of a single subunit; it showed activity with l-phenylalanine, phenylpyruvate, 4-hydroxyphenyl-pyruvate, indole-3-pyruvate and α-ketoisocaproate, but not with imidazolepyruvate, d-phenylalanine and other l-amino acids tested. Maximum activities with phenylpyruvate (310 μmol min-1 mg-1 of purified protein) were observed at pH 10 and 53°C. Sorbitol and glycerol stabilized the enzyme.
Similar content being viewed by others
Abbreviations
- RuMP:
-
ribulose monophosphate
- HPS:
-
hexulose-6-phosphate synthase
- HPT:
-
hexulose-6-phosphate isomerase
- FPLC:
-
fast protein liquid chromatography
References
AsanoY, EndoK (1988) Amino acid racemase with broad substrate specificity, its properties and use in phenylalanine racemization. Appl Microbiol Biotechnol 29: 523–527
AsanoY, NakazawaA (1987) High yield synthesis of l-amino acids by phenylalanine dehydrogenase from Sporosarcina ureae. Agr Biol Chem 51: 2035–2036
AsanoY, NakazawaA, EndoK (1987a) Novel phenylalanine dehydrogenase from Sporosarcina ureae and Bacillus sphaericus. Purification and characterization. J Biol Chem 262: 10346–10354
AsanoY, NakazawaA, EndoK, HibinoY, OhmoriM, NumaoN, KondoK (1987b) Phenylalanine dehydrogenase of Bacillus badius. Purification, characterization and gene cloning. Eur J Biochem 168: 153–159
BoerLde, HarderW, DijkhuizenL (1988) Phenylalanine and tyrosine metabolism in the facultative methylotroph Nocardia sp. 239. Arch Microbiol 149: 459–465
BoerLde, VrijbloedJW, GrobbenG, DijkhuizenL (1989) Regulation of aromatic amino acid biosynthesis in the ribulose monophosphate cycle methylotroph Nocardia sp. 239. Arch Microbiol 151: 319–325
BradfordMM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254
CampagnaR, BückmannAF (1987) Comparison of the production of intracellular l-phenylalanine dehydrogenase by Rhodococcus species M4 and Sporosarcina ureae at 50 liter scale. Appl Microbiol Biotechnol 26: 417–421
DuineJA, FrankJ, BerkhoutMPJ (1984) NAD-dependent, PQQ-containing methanol dehydrogenase: a bacterial dehydrogenase in a multienzyme complex. FEBS Lett 168: 217–221
EvansCT, BellamyW, GleesonM, AokiH, HannaK, PetersonW, ConradD, MisawaM (1987) A novel, efficient biotransformation for the production of l-phenylalanine. Bio/Technology 5: 818–823
HarderW, VisserK, KuenenJG (1974) Laboratory fermenter with an improved magnetic drive. Lab Pract 23: 644–645
HazeuW, BruynJCde, DijkenJPvan (1983) Nocardia sp. 239, a facultative methanol utilizer with the ribulose monophosphate pathway of formaldehyde fixation. Arch Microbiol 135: 205–210
HummelW, WeissN, KulaMR (1984) Isolation and characterization of a bacterium possessing l-phenylalanine dehydrogenase activity. Arch Microbiol 137: 47–52
HummelW, SchmidtE, WandreyC, KulaMR (1986) l-Phenylalanine dehydrogenase from Brevibacterium sp. for production of l-phenylalanine by reductive amination of phenylpyruvate. Appl Microbiol Biotechnol 25: 175–185
HummelW, SchütteH, SchmidtE, WandreyC, KulaMR (1987) Isolation of l-phenylalanine dehydrogenase from Rhodococcus sp. M4 and its application for the production of l-phenylalanine. Appl Microbiol Biotechnol 26: 409–416
JohnsonML, FrasierSG (1985) Non linear least squares analysis. Meth Enzymol 117: 301–342
LaemmliUK, FavreK (1973) Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol 80: 575–599
LeveringPR, vanDijkenJP, VeenhuisM, HarderW (1981) Arthrobacter P1, a fast growing methylotroph with amine oxidase as a key enzyme in the metabolism of methylated amines. Arch Microbiol 129: 72–78
LeveringPR, DijkhuizenL (1985) Regulation of methylotrophic and heterotrophic metabolism in Arthrobacter P1. Growth on mixtures of methylamine and acetate in batch and continuous cultures. Arch Microbiol 142: 113–120
MatsunagaT, HigashijimaM, NakatsugawaH, NishimuraS, KitamuraT, TsujiM, KawaguchiT (1987) Conversion of phenylpyruvate to l-phenylalanine by immobilized Clostridium butyricum-alanine dehydrogenase-Micrococcus luteus under hydrogen high pressure. Appl Microbiol Biotech 27: 11–14
MisonoH, YonezawaJ, NagataS, NagasakiS (1989) Purification and characterization of a dimeric phenylalanine dehydrogenase from Rhodococcus maris K-18. J Bacteriol 171: 33–36
SchuttenI, HarderW, DijkhuizenL (1987) Thermostability of l-phenylalanine aminotransferase from thermophilic bacteria. Appl Microbiol Biotechnol 27: 292–298
VishniacW, SanterM (1957) The thiobacilli. Bacteriol Rev 21: 195–213
WrayW, BoulikasT, WrayVP, HancockR (1981) Silver staining of proteins in polyacrylamide gels. Anal Biochem 118: 197–203
YonahaK, SodaK (1986) Applications of stereoselectivity of enzymes: synthesis of optically active amino acids and α-hydroxy acids, and stereospecific isotope-labeling of amino acids, amines and coenzymes. Adv Biochem Eng Biotechnol 33: 95–130
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
de Boer, L., van Rijssel, M., Euverink, G.J. et al. Purification, characterization and regulation of a monomeric l-phenylalanine dehydrogenase from the facultative methylotroph Nocardia sp. 239. Arch. Microbiol. 153, 12–18 (1989). https://doi.org/10.1007/BF00277534
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00277534