Abstract
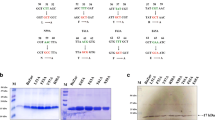
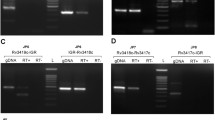
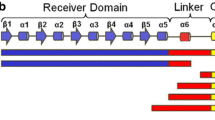
The transcription factor ALCR of the ethanol utilisation pathway in Aspergillus nidulans contains a zinc binuclear motif (CysX2CysX6CYSX16CysX2CysX6 Cys), within the DNA-binding domain located in the N-terminal region of the ALCR protein. Specific targets have been localised in the promoter of the alcR gene, involved in the autoregulation process, and in the promoter of the structural gene alcA (encoding alcohol dehydrogenase I), which is also under the control of ALCR. The DNA-binding domain has been expressed in-Escherichia coli as a GST-ALCR (7–58*) fusion protein and also obtained as an ALCR (7–58*) peptide. Both the ALCR fusion protein and the ALCR peptide are able to bind 65Zn(II) in vitro, if reduction of cysteines occurs prior to the addition of zinc. Competition experiments showed that Cd(II), Co(II) and Cu(II) are efficient competitors for the zinc binding sites. The ALCR DNA-binding domain was shown to contain 2 mol of tightly bound Zn(II) per mole of fusion protein. Removal of the intrinsic Zn(II) requires treatment with Chelex. This treatment abolishes the ability of the protein to bind to the targets of ALCR located in the alcA and alcR promoters. The apo-ALCR DNA-binding motif could be reconstituted with Zn(II) or Cd(II), restoring specific DNA binding to both types of targets. Thus a direct relationship was shown to exist between the zinc content of ALCR and its DNA-binding activity.
Similar content being viewed by others
References
Baleja JD, Marmorstein R, Harrison SC, Wagner G (1992) Solution structure of the DNA-binding domain of the Cd2-GAL4 from S. cerevisiae. Nature 356:450–453
Felenbok B (1991) The ethanol utilization regulon of Aspergillus nidulans: the alcA-alcR system as a tool for the expression of recombinant proteins. J Biotechnol 17:11–18
Felenbok B, Sequeval D, Mathieu M, Sibley S, Gwynne DI, Davies RW (1988) The ethanol regulon in Aspergillus nidulans: Characterization and sequence of the positive regulatory gene alcR. Gene 73:385–396
Felenbok B, Sequeval D, Judewicz N, Mathieu M, Lenouvel F, Prangé T, Scazzocchio C, Dowzer C, Kelly J, Kulmburg P (1991) Control of the ethanol regulon in Aspergillus nidulans. In: Stahl U, Tudzynski P (eds) Proceedings of the 4th EMBO meeting on molecular biology of filamentous fungi. VCH, pp 167–176
Flavio Della Seta (1990) Le facteur ABFI de Saccharomyces cerevisiae et son interaction avec les gènes RPC40, RPC160, L2A et L2B. Purification, reconnaissance de l'ADN et rôle dans la transcription. Thesis, Université Paris-Sud XI, Orsay, France
Freemont PS, Lane AN, Sanderson MR (1991) Structural aspects of protein-DNA recognition. Biochem J 278:1–23
Grossman SR, Laimins LL (1989) E6 protein of human papillomavirus type 18 binds zinc. Oncogene 4:1089–1093
Gwynne DI, Buxton FP, Sibley S, Davies RW, Lockington RA, Scazzocchio C, Sealy-Lewis HM (1987) Comparison of the cis-acting control regions of two coordinately controlled genes involved in ethanol utilization in Aspergillus nidulans. Gene 51:205–216
Halvorsen YDC, Nandabalan K, Dickson RC (1990) LAC9 DNA-binding domain coordinates two zinc atoms per monomer and contacts DNA as a dimer. J Biol Chem 265:13283–13289
Johnson PF, McKnight SL (1989) Eukaryotic transcriptional regulatory proteins. Annu Rev Biochem 58:799–839
Johnston M (1987) Genetic evidence that zinc is an essential cofactor in the DNA binding domain of GAL4 protein. Nature 328:353–355
Kraulis PJ, Raine ARC, Gadhavi PL, Laue ED (1992) Structure of the DNA-binding domain of zinc GAL4. Nature 356:448–450
Kulmburg P, Prangé T, Mathieu M, Sequeval D, Scazzocchio C, Felenbok B (1991) Correct intron splicing generates a new type of a putative zinc-binding domain in a transcriptional activator of Aspergillus nidulans. FEBS Lett 280:11–16
Kulmburg P, Sequeval D, Lenouvel F, Mathieu M, Felenbok B (1992a) Identification of the promoter region involved in the autoregulation of the transcriptional activator ALCR in Aspergillus nidulans. Mol Cell Biol 12:1932–1939
Kulmburg P, Judewicz N, Mathieu M, Lenouvel F, Sequeval D, Felenbok B (1992b) Specific binding sites for the activator protein, ALCR, in the alcA promoter of the ethanol regulon of Aspergillus nidulans. J Biol Chem 267:21146–21153
Kulmburg P, Mathieu M, Dowzer C, Kelly J, Felenbok B (1993) Specific binding sites in the alcR and alcA promoters of the ethanol regulon for the CREA repressor mediating carbon catabolite repression in Aspergillus nidulans. Mol Microbiol 7:847–857
Lockington RA, Sealy-Lewis HM, Scazzocchio C, Davies RW (1985) Cloning and characterization of the ethanol utilization regulon in Aspergillus nidulans. Gene 33:137–149
Lockington RA, Scazzocchio C, Sequeval D, Mathieu M, Felenbok B (1987) Regulation of alcR, the positive regulatory gene of the ethanol utilization regulon of Aspergillus nidulans. Mol Microbiol 1:275–281
Marmorstein R, Carey M, Ptashne M, Harrison SC (1992) DNA recognition by GAL4: structure of a protein-DNA complex. Nature 356:408–414
Pan T, Coleman JE (1990) The DNA binding domain of GAL4 forms a binuclear metal ion complex. Biochemistry 29:3023–3029
Pateman JH, Doy CH, Olson JE, Norris U, Creaser EH, Hynes M (1983) Regulation of alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (AldDH) in Aspergillus nidulans. Proc R Soc Lond [biol] 217:243–264
Pickett M, Gwynne DI, Buxton FP, Elliott R, Davies RW, Lockington RA, Scazzocchio C, Sealy-Lewis HM (1987) Cloning and characterization of the aldA gene of Aspergillus. Gene 51:217–226
Povey JF, Diakun GP, Garner CD, Wilson SP, Laue ED (1990) Metal ion co-ordination in the DNA binding domain of the yeast transcriptional activator GAL4. FEBS Lett 266:142–146
Schiff LA, Nibert ML, Fields BN (1988) Characterization of a zinc blotting technique: Evidence that a retroviral gag protein binds zinc. Proc Natl Acad Sci USA 85:4195–4199
Smith DB, Johnson KS (1988) Single step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene 67:31–40
Treich I, Riva M, Sentenac A (1991) Zinc-binding subunits of yeast RNA polymerases. J Biol Chem 266:21971–21976
Vallee BL, Galdes A (1984) The metallobiochemistry of zinc enzymes. Adv Enzymol 56:283–431
Author information
Authors and Affiliations
Additional information
Communicated by C. van den Hondel
Rights and permissions
About this article
Cite this article
Sequeval, D., Felenbok, B. Relationship between zinc content and DNA-binding activity of the DNA-binding motif of the transcription factor ALCR in Aspergillus nidulans . Molec. Gen. Genet. 242, 33–39 (1994). https://doi.org/10.1007/BF00277345
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00277345