Abstract
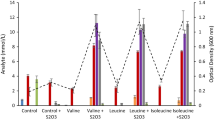
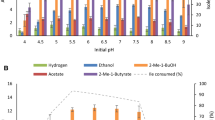
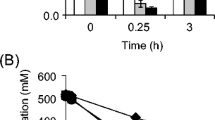
The hyperthermophilic archaeon Pyrococcus furiosus was found to form substantial amounts of l-alanine during batch growth on either cellobiose, maltose or pyruvate. Acetate, CO2 and H2 were produced next to alanine. The carbon- and electron balances were complete for all three substrates. Under standard growth conditions (N2/CO2 atmosphere) an alanine/acetate ratio of about 0.3 was found for either substrate. The alanine /acetate ratio was influenced, however, by the hydrogen partial pressure. In the presence of S0 or in coculture with Methanococcus jannaschii this ratio was only 0.07, whereas under a H2/CO2 atmosphere this ratio could amount up to 0.8. Alanine formation was also aflected by the NH sup+inf4 concentration, i.e. below 4 mM, NH sup+inf4 becomes limiting to alanine formation. Alanine formation was shown to occur via an alanine aminotransferase, which exhibited a specific activity in cell-free extract of up to 6.0 U/mg (90°C; direction of pyruvate formation). The alanine aminotransferase probably cooperates with glutamate dehydrogenase (up to 23 U/mg; 90°C) and ferredoxin: NADP+ oxidoreductase (up to 0.7 U/mg, using methyl viologen; 90°C) to recycle the electron acceptors involved in catabolism. Thus, the existence of this unusual alanine-forming branch enables P. furiosus to adjust its fermentation, depending on the redox potential of the terminal electron acceptor.
Similar content being viewed by others
Abbreviations
- DTT:
-
dithiothreitol
- MV:
-
methyl viologen
- AAT:
-
alanine aminotransferase
- GDH:
-
glutamate dehydrogenase
- MV: NADP+ OR:
-
methyl viologen: NADP+ oxidoreductase
References
AdamsMWW (1990) The metabolism of hydrogen by extreme thermophilic, sulfur dependent archaebacteria. FEMS Microbiol Rev 75: 219–238
AonoS, BryantFO, AdamsMWW (1989) A novel and remarkably thermostable ferredoxin from the hyperthermophilic archaebacterium Pyrococcus furiosus. J Bacteriol 171: 3433–3439
BlumentalsII, ItohM, OlsenGJ, KellyRM (1990) Role of polysulfides in reduction of elemental sulfur by the hyperthermophilic archaebacterium Pyrococcus furiosus. Appl Environ Microbiol 56: 1255–1262
Bonch-OsmolovskayaEA, StetterKO (1991) Interspecies hydrogen transfer in cocultures of thermophilic Archaea. Syst Appl Microbiol 14: 205–208
BradfordMM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254
BryantFO, AdamsMWW (1989) Characterization of hydrogenase from the hyperthermophilic archaebacterium, Pyrococcus furiosus. J Biol Chem 264: 5070–5079
ConsalviV, ChiaraluceR, PolitiL, VaccaroR, DeRosaM, ScandurraR (1991) Extremely thermostable glutamate dehydrogenase from the hyperthermophilic archaebacterium Pyrococcus furiosus. Eur J Biochem 202: 1189–1196
EdwardsMR, GilroyFV, JimenezMB, O'SullivanWJ (1989) Alanine is a major end product of metabolism by Giardial lamblia: a proton nuclear magnetic resonance study. Mol Biochem Parasitol 37: 19–26
FialaG, StetterKO (1986) Pyrococcus furiosus sp. nov. represents a novel genus of marine heterotrophic archaebacteria growing optimally at 100°C. Arch Microbiol 145: 56–61
GottschalkG (1986) Bacterial metabolism, 2nd edn. Springer, Berlin Heidelberg New York Tokyo
IanottiEL, KafkewitzD, WolinMJ, BryantMP (1973) Glucose fermentation products of Ruminococcus albus in continuous culture with Vibrio succinogenes: changes caused by interspecies transfer of H2. J Bacteriol 114: 1231–1240
JonesWJ, LeighJA, MayerF, WoeseCR, WolfeRS (1983) Methanococcus jannaschii sp. nov., an extremely thermophilic methanogen from a submarine hydrothermal vent. Arch Microbiol 136: 254–261
KengenSWM, LuesinkEJ, StamsAJM, ZehnderAJB (1993) Purification and characterization of an extremely thermostable β-glucosidase from the hyperthermophilic archaeon Pyrococcus furiosus. Eur J Biochem 213: 305–312
LamedRJ, LobosJH, SuTM (1988) Effects of stirring and hydrogen on fermentation products of Clostridium thermocellum. Appl Environ Microbiol 54: 1216–1221
LowryOH, RosebroughNJ, FarrA, RandallRJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275
MalikB, SuWW, WaldHL, BlumentalsII, KellyRM (1989) Growth and gas production for hyperthermophilic archaebacterium, Pyrococcus furiosus. Biotechnol Bioeng 34: 1050–1057
McCartyPL (1975) Stoichiometry of biological reactions. Prog Water Technol 7: 157–170
MukundS, AdamsMWW (1990) Characterization of a tungsteniron-sulfur protein exhibiting novel spectroscopic and redox properties from the hyperthermophilic archaebacterium Pyrococcus furiosus. J Biol Chem 265: 11508–11516
MukundS, AdamsMWW (1991) The novel tungsten-iron-sulfur protein of the hyperthermophilic archaebacterium, Pyrococcus furiosus, is an aldehyde ferredoxin oxidoreductase. J Biol Chem 266: 14208–14216
PagetTA, RaynerMH, ShippDWE, LloydD (1990) Giardia lamblia produces alanine anaerobically but not in the presence of oxygen. Mol Biochem Parasitol 42: 63–68
ParkJB, FanC, HoffmanBM, AdamsMWW (1991) Potentiometric and electron nuclear double resonance properties of the two spin forms of the [4 Fe−4 S]+ cluster in the novel ferredoxin from the hyperthermophilic archaebacterium Pyrococcus furiosus. J Biol Chem 266: 19351–19356
RobbFT, ParkJB, AdamsMWW (1992) Characterization of an extremely thermostable glutamate dehydrogenase: a key enzyme in the primary metabolism of the hyperthermophilic archaebacterium Pyrococcus furiosus. Biochim Biophys Acta 1120: 267–272
SchäferT, SchönheitP (1991) Pyruvate metabolism of the hyperthermophilic archaebacterium Pyrococcus furiosus. Arch Microbiol 155: 366–377
SchäferT, SchönheitP (1992) Maltose fermentation to acetate, CO2 and H2 in the anaerobic hyperthermophilic archaeon Pyrococcus furiosus: evidence for the operation of a novel sugar fermentation pathway. Arch Microbiol 158: 188–202
SchäferT, SchönheitP (1993) Gluconeogenesis from pyruvate in the hyperthermophilic archaeon Pyrococcus furiosus: involvement of reactions of the Embden-Meyerhof pathway. Arch Microbiol 159: 354–363
SchichoRN, SnowdenLJ, MukundS, ParkJB, AdamsMWW, KellyRM (1993a) Influence of tungsten on metabolic patterns in Pyrococcus furiosus, a hyperthermophilic archaeon. Arch Microbiol 159: 380–385
SchichoRN, MaK, AdamsMWW, KellyRM (1993b) Bioenergetics of sulfur reduction in the hyperthermophilic archaeon Pyrococcus furiosus. J Bacteriol 175: 1823–1830
StamsAJM, DijkJBvan, DijkemaC, PluggeCM (1993) Growth of syntrophic propionate oxidizing bacteria with fumarate in the absence of methanogenic bacteria. Appl Environ Microbiol 59: 1114–1119
StetterKO, FialaG, HuberG, HuberR, SegererA (1990) Hyperthermophilic microorganisms. FEMS Microbiol Lett 75: 117–124
ThauerRK, JungermannK, DeckerK (1977) Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev 41: 100–180
UhlenbuschI, SahmH, SprengerGA (1991) Expression of an l-alanine dehydrogenase gene in Zymomonas mobilis and excretion of l-alanine. Appl Environ Microbiol 57: 1360–1366
WeimerPJ, ZeikusJG (1977) Fermentation of cellulose and cellobiose by Clostridium thermocellum in the absence and presence of Methanobacterium thermoautotrophicum. Appl Environ Microbiol 33: 289–297
WolinEA, WolinMJ, WolfeRS (1963) Formation of methane by bacterial extracts. J Biol Chem 238: 2882–2886
ZehnderAJB, HuserBA, BrockTD, WuhrmannK (1980) Characterization of an acetate-decarboxylating, non-hydrogen-oxidizing methane bacterium. Arch Microbiol 124: 1–11
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kengen, S.W.M., Stams, A.J.M. Formation of l-alanine as a reduced end product in carbohydrate fermentation by the hyperthermophilic archaeon Pyrococcus furiosus . Arch. Microbiol. 161, 168–175 (1994). https://doi.org/10.1007/BF00276479
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00276479