Abstract
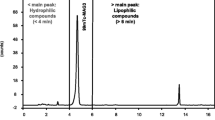
The ionic impurities present in 113mIn-colloid may be chelated with Ca-EDTA (calcium ethylenediamine tetraacetic acid) and easily separated by paper chromatography using methanol eluent. The procedure does not exceed 5 min and is thus suitable for quality control of 113mIn-colloid prior to patient administration.
Similar content being viewed by others
References
Colombetti LG, Moerlien S, Patel GO (1976) Rapid determination of oxidation state of unbound 99mTc and labeling yield in 99mTc-labeled radiopharmaceuticals. J Nucl Med 17:805–809
Colombetti LG, Goodwin DA, Hermanson R (1969) 113mIn-labeled compound for liver and spleen studies. J Nucl Med 10:597–602
Czechoslovak Pharmacopoeia (1970) Third Edition, Prague
Goodwin DA, Stern HS, Wagner HN, Jr (1966) A new radiopharmaceutical for liver scanning. Nucleonics 24:65
Zimmer AM, Pavel DG (1977) Rapid miniaturized chromatographic quality-control procedures for Tc-99m radiopharmaceuticals. J Nucl Med 18:1230–1233
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Komárek, P., Křivský, Z., Oppelt, A. et al. Quality control of 113mIn-colloid: A new rapid method. Eur J Nucl Med 7, 37–38 (1982). https://doi.org/10.1007/BF00275243
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00275243