Summary
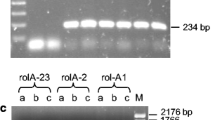
Brassica napus and Brassica juncea were infected with a number of Agrobacterium tumefaciens strains. Tumourigenesis was very rapid and extremely efficient on B. juncea with all but one of the strains. Tumourigenesis on B. napus varied widely. It was very efficient with the nopaline strains, was reduced with the succinamopine strain A281 and was very weak with the octopine strains. The latter observation was confirmed with six different B. napus rapeseed cultivars. The selectivity was due to differences in the virulence of Ti plasmids with B. napus, rather than the tumourigenicity of the T-DNA or virulence of the chromosomal genes associated with the strains. An exception was strain LBA4404. The virulence of the octopine strains was increased by coinfection with more virulent disarmed strains and by induction with acetosyringone.
Similar content being viewed by others
References
Anonymous (1988) Canola Growers Manual. Canola Council of Canada. Winnipeg, Canada, 1424 pp
Binns AN, Thomashow MF (1988) Cell biology of Agrobacterium infection and transformation of plants. Ann. Rev. Microbiol, 42: 575–606
Charest PJ, Dion P (1985) The influence of temperature on tumourigenesis induced by various strains of Agrobacterium. Can J Bot 63:1160–1167
Charest PJ, (1988) Genetic transformation of Brassica napus via Agrobacterium spp. and the development of herbicide resistance. PhD thesis, Dep't of Biology, Carleton University, Ottawa, Canada
Charest PJ, Holbrook LA, Gabard J, Iyer VN, Miki BL (1988) Agrobacterium-mediated transformation of thin cell layer explants from Brassica napus. L. Theor Appl Genet 75:438–445
DeCleene M, DeLey J (1976) The host range of crown gall. Bot Rev 42:389–466
Figurski DH, Helinski DR (1979) Replication of an origin containing derivative of plasmid RK2 dependant on a plasmid function provided in trans. Proc Natl Acad Sci USA 76: 1648–1652
Fry J, Barnason A, Horsh RB (1987) Transformation of Brassica napus with Agrobacterium tumefaciens based vectors. Plant Cell Rep 6: 321–325
Hamel A (1987) Involvement of the chromosomal recA gene of Agrobacterium tumefaciens in the processing of the DNA of its tumor-inducing plasmid. M.Sc. Thesis Carleton University 100 pp
Hoekema A, Hirsch PR, Hooykaas PJJ, Schilperoort RA (1983) A binary plant vector strategy based on separation of VIR-and T-region of the Agrobacterium tumefaciens Ti-plasmid. Nature 303: 179–180
Holbrook LA, Haffner M, Miki BL (1986) A sensitive fluorographic method for the detection of nopaline and octopine synthase activities in Brassica crown-gall tissues. Biochem Cell Biol 64: 126–132
Holbrook LA, Miki BL (1985) Brassica crown gall tumorigenesis and in vitro culture of transformed tissue. Plant Cell Rep. 4:329–332
Holsters M, Silva B, Vliet F Van, Genetello C, Block M de, Dhaese P, Depicker A, Inze D, Engler G, Villaroel R, Montagu M Van, Schell J (1980) The functional organization of the nopaline Agrobacterium tumefaciens pTi C58. Plasmid 3:212–230
Iyer VN, Klee HJ, Nester EW (1982) Units of genetic expression in the virulence region of a plant tumor-inducing plasmid of Agrobacterium tumefaciens. Mol Gen Genet 188: 418–424
Koncz C, Schell J (1986) The promoter of T L -DNA gene 5 controls the tissue-specific expression of chimaeric genes carried by a novel type of Agrobacterium binary vector. Mol Gen Genet 204: 383–396
Martens JW, Seaman WL, Atkinson TG (1984) Diseases of Canola, rapeseed, and mustard in: Diseases of field crops in Canada. The Canadian Phytopathological Society pp. 116–121
Matthews VH, Bhatia CR, Mitra R, Krishna TG, Rao PS (1985) Regeneration of shoots from Brassica juncea (Linn) Czern and Coss cells transformed by Agrobacterium tumefaciens and expression of nopaline dehydrogenase genes. Plant Sci 39:49–54
Matthews H, Rao PS, Bhatia CR (1986) Transformation of Brassica juncea by Agrobacterium tumefaciens harbouring plasmid pTiT37 and its rooty mutant pTiT37.14a/a. J Genet 65: 37–44
Ohlsson M, Erickson T (1988) Transformation of Brassica campestris protoplasts with Agrobacterium tumefaciens. Hereditas 108: 173–177
Coms G, Hooykaas PJJ, Van Veen RJM, Van P, Beelen, Regensburg-Twink TGJ, Schilperoort RA (1982) Octopine Ti plasmid deletion mutants of Agrobacterium tumefaciens with emphasis on the right side of the T-region. Plasmid 7: 15–29
Otten L, Greve H DE, Leemans J, Hain R, Hooykaas PJJ (1984) Restoration of virulence of VIR region mutants of Agrobacterium tumefaciens strain B6S3 by coinfection with normal and mutant Agrobacterium strains. Mol Gen Genet 195:159–163
Otten L, Piotroviak G, Hooykaas P, Dubois P, Szegedi E, Schell J (1985) Identification of an Agrobacterium tumefaciens pTiB63S3 VIR region fragment that enhances the virulence of pTiC58. Mol Gen Genet 199: 189–193
Petit A, Tempe J (1978) Isolation of Agrobacterium Ti plasmid regulatory mutants. Mol Gen Genet 166:147–155
Pua EC, Mehra-Palta A, Nagy F, Chua NH (1987) Transgenic plants of Brassica napus. L. BioTechnology 5:815–817
Radke SE, Andrews BM, Moloney MM, Crouch ML, Kridl JC, Knauf VC (1988) Transformation of B. napus L. using Agrobacterium tumefaciens: developmentally regulated expression of a reintroduced napin gene. Theor Appl Genet 75: 685–694
Ream LW, Gordon MP, Nester EW (1983) Multiple mutations in the T region of the Agrobacterium tumefaciens tumor-inducing plasmid. Proc Natl Acad Sci USA 80: 1660–1664
Rogers SG, Klee H (1987) Pathways to plant genetic engineering in Plant DNA infectious agents. T Hohn and J Schell eds. Springer-Verlag New York pp. 179–203
Sciaky D, Montoya AL, Chilton MD (1978) Fingerprints of Agrobacterium Ti plasmids. Plasmid 1: 238–253
Van Haute E, Joos H, Maes M, Warren G, VanMontagu M, Schell J (1983) Intergeneric transfer and exhange recombination of restriction fragments cloned in pBR322: a novel strategy for the reversed genetics of the Ti plasmids of Agrobacterium tumefaciens EMBO J 2:411–417
Zambryski P, Joos H, Genetello C, Leemans J, Van Montagu M, Schell J (1983) Ti plasmid vector for the introduction of DNA into plant cells without alteration of their normal regeneration capacity. EMBO J 2: 2143–2150
Author information
Authors and Affiliations
Additional information
Communicated by F. Constabel
Rights and permissions
About this article
Cite this article
Charest, P.J., Iyer, V.N. & Miki, B.L. Virulence of Agrobacterium tumefaciens strains with Brassica napus and Brassica juncea . Plant Cell Reports 8, 303–306 (1989). https://doi.org/10.1007/BF00274136
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00274136