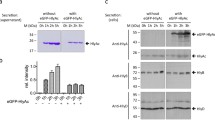
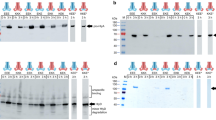
Summary
We studied the efficiency of the pHly152-derived haemolysin transport system using PhoA-HlyA fusion proteins and different constructs which provide HlyB/HlyD in trans. The optimal C-terminal HlyA signal consists of the last 60 amino acids. Longer stretches of HlyA do not improve the transport efficiency of PhoA-HlyA fusion proteins. The introduction of deletions and/or replacements in the 60 amino acid HlyA signal domain revealed at least three functional regions with different degrees of specificity. Amino acids 1–21 (numbered from the N-terminal part of the 60 amino acid HlyA signal), termed region I, could be replaced by a Pro-containing peptide. The other two regions II and III (amino acids 22–40 and 41–60, respectively) seem to interact directly with the HlyB/HlyD translocator since a PhoA fusion protein which contains either of the two regions was still secreted in a HlyB/HlyD-dependent mode, albeit at low efficiency. An efficient trans-complementing HlyB/HlyD system was only obtained from the pHLy152-encoded hly determinant when the regulatory hlyR element was provided in cis. Secretion of the PhoA-HlyA fusion protein did not interfere with the secretion of HlyA even when the fusion protein was induced to a high level. This suggests that the capacity of the HlyB/HlyD translocation system is high and not normally saturated by its natural HlyA substrate.
Similar content being viewed by others
References
Baty D, Lloubes R, Geli V, Lazdunski C, Howard SP (1987) Extracellular release of colicin A is non-specific. EMBO J 6:2463–2468
Boyer HW, Roulland-Dussoix D (1969) A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol 41:459–472
Carter-Muenchau P, Wolf RE Jr (1987) A method for cloning mixtures of long, synthetic oligonucleotides. Gen Anal Techn 4:105–110
Eisenberg D, Weiss RM, Terwilliger TC (1982) The helical hydrophobic moment: a measure of the amphiphilicity of a helix. Nature 299:371–374
Erb K, Vogel M, Wagner W, Goebel W (1987) Alkaline phosphatase which lacks its own signal sequence becomes enzymatically active when fused to N-terminal sequences of Escherichia coli haemolysin (HlyA). Mol Gen Genet 208:88–93
Felmlee T, Welch RA (1988) Alterations of amino acid repeats in the Escherichia coli hemolysin affect cytolytic activity and secretion. Proc Natl Acad Sci USA 85:5269–5273
Garnier J, Osguthorpe DJ, Robson B (1978) Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol 120:97–120
Gentschev I, Hess J, Goebel W (1990) Change in the cellular localization of alkaline phosphatase by alteration of its carboxyterminal sequence. Mol Gen Genet 222:211–216
Goebel W, Hedgpeth J (1982) Cloning and functional characterization of the plasmid-encoded hemolysin determinant of Escherichia coli. J Bacteriol 151:1290–1298
Härtlein M, Schießl S, Wagner W, Rdest U, Kreft J, Goebel W (1983) Transport of hemolysin by Escherichia coli. J Cell Biochem 22:87–97
Hess J (1990) Transport von Hämolysin in Escherichia coli. PhD thesis, University Würzburg, FRG
Hess J, Wels W, Vogel M, Goebel W (1986) Nucleotide sequence of a plasmid encoded hemolysin determinant and its comparison with a corresponding chromosomal hemolysin sequence. FEMS Microbiol Lett 34:1–11
Holland IB, Wang R, Seror SJ, Blight M (1989) Haemolysin secretion and other protein translocation mechanisms in gram-negative bacteria. In: Baumberg S, Hinster I, Rhodes M (eds) Microbial Products, New Approaches. Cambridge University Press, Cambridge, pp 219–254
Koronakis V, Cross M, Hughes C (1988) Expression of the E. coli hemolysin secretion gene hlyB involves transcript anti-termination within the hly operon. Nucleic Acids Res 16:4789–4800
Koronakis V, Koronakis E, Hughes C (1989) Isolation and analysis of the C-terminal signal directing export of Escherichia coli hemolysin across both bacterial membranes. EMBO J 8:595–605
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the heads of bacteriophage T4. Nature 227:680–685
Lee C, Beckwith J (1986) Cotranslational and post-translational protein translocation in procaryotic systems. Annu Rev Cell Biol 2:315–336
Ludwig A, Vogel M, Goebel W (1987) Mutations affecting activity and transport of haemolysin in E. coli. Mol Gen Genet 206:238–245
Mackman N, Nicaud JM, Gray L, Holland IB (1984) Functional characterization of a cloned haemolysin determinant from E. coli of human origin, encoding information for the secretion of a 107 K polypeptide. Mol Gen Genet 196:129–134
Mackman N, Baker K, Gray L, Haigh R, Nicaud JM, Holland B (1987) Release of a chimeric protein into the medium from Escherichia coli using the C-terminal secretion signal of haemolysin. EMBO J 6:2835–2841
Manoil C, Beckwith J (1985) TnPhoA: A transposon probe for protein export signals. Proc Natl Acad Sci USA 82:8129–8133
Nicaud JM, Mackman N, Gray L, Holland IB (1986) The C-terminal 23 kD peptide of Escherichia coli haemolysin 2001 contains all the information necessary for its secretion by the haemolysin (Hly) machinery. FEBS Lett 204:331–335
Oakley BR, Kirsch DR, Morris NR (1980) A simplified silver stain for detecting proteins in polyacrylamide gels. Anal Biochem 105:361–363
Oliver D, Beckwith J (1981) E. coli mutant pleiotropically defective in the export of secreted proteins. Cell 25:765–772
Oropeza-Wekerle RL, Müller E, Kern P, Meyermann R, Goebel W (1989) Synthesis, inactivation and localization of extracellular and intracellular Escherichia coli hemolysins. J Bacteriol 171:2783–2788
Oropeza-Wekerle RL, Speth W, Imhof B, Gentschev I, Goebel W (1990) Translocation and compartmentalization of Escherichia coli hemolysin (HlyA). J Bacteriol 172:3711–3717
Pohlner J, Halter R, Beyreuther K, Meyer TF (1987) Gene structure and extracellular secretion of Neisseria gonorrhoeae IgA protease. Nature 325:458–462
Pugsley AP, Reyss I (1990) Five genes at the 3′ end of the Klebsiella pneumoniae pulC operon are required for pullulanase secretion. Mol Microbiol, in press
Pugsley AP, Schwartz M (1985) Export and secretion of proteins by bacteria. FEMS Microbiol Rev 32:3–38
Randall LL, Hardy SJS, Thom JR (1987) Export of proteins: a biochemical view. Annu Rev Microbiol 41:507–541
Saier MH, Werner PK, Müller M (1989) Insertion of proteins into bacterial membranes: mechanism, characteristics and comparisons with the eucaryotic process. Microbiol Rev 53:333–366
Towbin H, Staehlin H, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76:4350–4354
Vogel M, Hess J, Then I, Juarez A, Goebel W (1988) Characterization of a sequence (hlyR) which enhances synthesis and secretion of hemolysin in Escherichia coli. Mol Gen Genet 212:76–84
Wagner W, Vogel M, Goebel W (1983) Transport of hemolysin across the outer membrane of Escherichia coli requires two functions. J Bacteriol 154:200–210
Walter P, Lingappa VR (1986) Mechanisms of protein translocation across the endoplasmic reticulum membrane. Annu Rev Cell Biol 2:499–516
Welch RA, Pellett S (1988) The transcriptional organization of the Escherichia coli hemolysin genes. J Bacteriol 170:1622–1630
Wolfe PB, Rice M, Wickner W (1985) Effects of two sec genes on protein assembly into the plasma membrane of Escherichia coli. J Biol Chem 260:1836–1841
Yanagida N, Uuzumi T, Beppu T (1986) Specific excretion of Serratia marcescens protease through the outer membrane of E. coli. J Bacteriol 166:937–944
Author information
Authors and Affiliations
Additional information
Communicated by E.K.F. Bautz
Dedicated to Prof., Dr. F. Lingens on the occasion of his 65th birthday
Rights and permissions
About this article
Cite this article
Hess, J., Gentschev, I., Goebel, W. et al. Analysis of the haemolysin secretion system by PhoA-HlyA fusion proteins. Molec. Gen. Genet. 224, 201–208 (1990). https://doi.org/10.1007/BF00271553
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00271553