Abstract
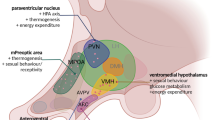
In order to determine the changes in gonadotrophin releasing hormone (GnRH) and dopaminergic activity within the brain during the onset of sexual precocity, a Halasz-like knife was developed to produce discrete parasagittal cuts in 2-week-old male broiler chicks. At 5 weeks of age, sexually precocious respondents were selected on the basis of advanced secondary sex characteristics and randomly paired with sham-operated controls. Each pair of birds was perfused with heparinized saline followed by 4% paraformaldehyde. Sections 40 μm thick, obtained throughout the hypothalamus, were immunostained with either anti-GnRH or anti-tyrosine hydroxylase (TH) to ascertain dopaminergic activity. Alternate sections from each pair of brains were also treated with cytochrome oxidase to determine metabolic activity levels or with Nissl stain to localize the knife cuts. Analysis revealed an increase in GnRH immunoreactivity within the bed nucleus of the pallial commissure (nCPa) and paraventricular nucleus (PVN), as well as the median eminence (ME). An increase in TH immunoreactivity was observed in the nucleus intramedialis (nI). Also an increase in metabolic activity was seen in the PVN as revealed by cytochrome oxidase reactivity. It is hypothesized that during the onset of puberty there is an increase in immunoreactive GnRH cell numbers as a result of a decrease in the inhibition of the GnRH system, possibly involving the nI and PVN. The source of the dopamine reported in the ME could be from the nI and other nearby nuclei. Dopamine from the tubero-infundibular area may be one of the putative neurotransmitters responsible for the increased activity of GnRH within the ME of chicks showing precocious puberty.
Similar content being viewed by others
References
Alvarez-Buylla A, Kirn JR, Nottebohm F (1990) Birth of projection neurons in adult avian brain may be related to perceptual or motor learning. Science 249:1444–1447
Barnes ND, Cloutier MD, Hayles AB (1963) The central nervous system and precocious puberty. In: Grumbach M, Grave G, Mayer F (eds) Control of the onset of puberty. Wiley, New York, pp 213–229
Blaehser S, King JA, Kuenzel WJ (1989) Testing of Arg-8-gonadotropin-releasing hormone-directed antisera by immunological and immunocytochemical methods for use in comparative studies. Histochemistry 93:39–48
Buonomo FC, Rabii J, Scaries CG (1981) Aminergic involvement in the control of luteinizing hormone secretion in the domestic fowl. Gen Comp Endocrinol 45:162–166
Davison BA, Kuenzel WJ (1991) Hypothalamic biogenic amine levels in broiler chicks showing advanced sexual maturation. Poultry Sci 70:1610–1618
Donovan BT, van der Werfften Bosch JJ (1959) The hypothalamus and sexual maturation in the rat. J Physiol 147:78–92
El Halawani ME, Burke WH, Ogren LA (1978) Effects of drugs that modify brain monoamine concentrations on photoperiodically-induced testicular growth in coturnix quail (Coturnix coturnix japonica). Biol Reprod 18:198–203
El Halawani ME, Burke WH, Ogren LA (1980) Involvement of catecholaminergic mechanisms in the photoperiodically induced rise in serum luteinizing hormone of Japanese quail (Coturnix coturnix japonica). Gen Comp Endocrinol 41:14–21
Foster RG, Panzica GC, Parry DM, Viglietti-Panzica C (1988) Immunocytochemical studies on the LHRH system of the Japanese quail: influence by photoperiod and aspects of sexual differentiation. Cell Tissue Res 253:327–335
Gellert RJ, Ganong WF (1960) Precocious puberty in rats with hypothalamic lesions. Acta Endocrinol 33:569–576
Gold RM, Kapatos G, Carey RJ (1973) A retracting wire knife for stereotaxic brain surgery made from a microliter syringe. Physiol Behav 10:813–815
Halasz B, Pupp L (1965) Hormone secretion of the anterior pituitary gland after physical interruption of all nervous pathways to the hypophysiotrophic area. J Endocrinol 77:553–562
Junier MP, Wolff A, Hoffman G, Costa ME, Ojeda SR (1990) Effect of hypothalamic lesions inducing precocious puberty on the morphological and functional maturation of the LHRH cell system. Soc Neurosci Abstr 16:322
Kalra SP, Crowley WR (1984) Norepinephrine-like effects of neuropeptide Y on LH release in the rat. Life Sci 35:1173–1176
King JA, Tobler CJ, Roeske RW, Day WA, Rivier JE, Millar RP (1983) A radioimmunoassay specific for [Gln8]LH-RH: application in the confirmation of the structure of chicken hypothalamic luteinizing hormone-releasing hormone. Peptides 4:883–887
Kuenzel WJ, Blaehser S (1991) The distribution of gonadotropin releasing hormone (GnRH) neurons and fibers throughout the chick brain (Gallus domesticus). Cell Tissue Res 264:481–495
Kuenzel WJ, Masson M (1988) A stereotaxic atlas of the brain of the chick (Gallus domesticus). Johns Hopkins Press, Baltimore, MD
Kuenzel WJ, Sharp PJ (1985) Parasagittal hypothalamic knife cuts in male chicks: advancement of reproductive function and changes in plasma concentrations of luteinizing hormone and androgen. Br Poultry Sci 26:199–205
Kuenzel WJ, Kirtinitis J, Saidel WS (1992) Comparison of tyrosine hydroxylase (TH) vs. dopamine (DA) specific antibody procedures for mapping DA-containing perikarya throughout the chick brain. Soc Neurosci Abstr 18:329
Mass JH, Kuenzel WJ (1983) Precocious development of the testes in chicks following parasagittal knife cuts of the lateral hypothalamic area. Dev Brain Res 10:165–169
Reiner A, Karten HJ, Brecha NC (1982) Enkephalin-mediated basal ganglia influences over the optic tectum: immunohistochemistry of the tectum and the spiriform lateral nucleus in pigeon. J Comp Neurol 208:37–53
Rohree H, Acheson AL, Thibault J, Thoenen H (1986) Developmental potential of quail dorsal root ganglion cells analyzed in vitro and in vivo. J Neurosci 6:2616–2624
Sawchenko PE, Swanson LW, Grzanna R, Howe PRC, Bloom SR, Polak JM (1985) Colocalization of neuropeptide-Y immunoreactivity in brainstem catecholaminergic neurons that project to the paraventricular nucleus of the hypothalamus. J Comp Neurol 241:138–153
Scanes CG, Rabii J, Buonomo F (1982) Brain amines and the regulation of anterior pituitary function in the domestic fowl. In: Scanes CG et al. (eds) Aspects of avian endocrinology: practical and theoretical implications. Graduate Studies, Texas Technological University, Lubbock, Texas, pp 13–31
Sclafani A (1971) Neural pathways involved in the ventromedial hypothalamic lesion syndrome in the rat. J Comp Physiol Psychol 77:70–96
Siegel S (1956) Nonparametric statistics for the behavioral sciences. In: Halow HF (ed) McGraw-Hill, New York
Sharp PJ, Follet BK (1969) The effect of hypothalamic lesions on gonadotropin release in Japanese quail. Neuroendocrinology 5:205–218
Sterling RJ, Sharp PJ (1982) The location of LH-RH neurones in the diencephalon of the domestic hen. Cell Tissue Res 222:283–298
Sutton SW (1988a) Neuropeptide Y (NPY): a possible role in the initiation of puberty. Endocrinology 123:2152–2154
Sutton SW (1988b) Evidence that neuropeptide Y (NPY) released into the hypophysial portal circulation participates in priming gonadotropes to the effects of gonadotropin releasing hormone (GnRH). Endocrinology 123:1208–1210
Swanson R, Sawchenko PE (1983) Hypothalamic integration: organization of the parvaventricular and supraoptic nuclei. Annu Rev Neurosci 6:269–324
Wong-Riley MTT (1979) Cytochrome oxidase: an endogenous metabolic marker for neuronal activity. Brain Res 171:11–28
Wray S, Hoffman G (1986) Catecholamine innervation of LH-RH neurons: a developmental study. Brain Res 399:327–331
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fraley, G.S., Kuenzel, W.J. Immunocytochemical and histochemical analyses of gonadotrophin releasing hormone, tyrosine hydroxylase, and cytochrome oxidase reactivity within the hypothalamus of chicks showing early sexual maturation. Histochemistry 99, 221–229 (1993). https://doi.org/10.1007/BF00269140
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00269140