Abstract
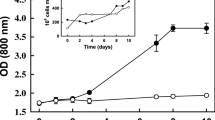
Chlorophyllous, heterotrophic periwinkle (Catharanthus roseus (L.) G. Don) cells were capable of sustained photoautotrophic growth in sugar-free B5 medium containing naphthaleneacetic acid and kinetin when provided with a CO2-enriched atmosphere. An increase in cell fresh weight, first observed approximately 2 weeks after transfer from heterotrophic to photoautotrophic conditions, coincided with the development of maximum chlorophyll content and photosynthetic activity. Electron micrographs revealed that chloroplasts of cells cultured photoautotrophically in continuous light contained large starch granules and exhibited a less extensive thylakoid system than did periwinkle mesophyll chloroplasts. Photoautotrophic cells did not accumulate vindoline or dimeric alkaloids.
Similar content being viewed by others
Abbreviations
- Chl:
-
chlorophyll
- dry wt:
-
dry weight
- fr wt:
-
fresh weight
- 2,4-D:
-
2,4-dichlorophenoxyacetic acid
- NAA:
-
naphthaleneacetic acid
References
Arnon DI (1949) Plant Physiol. 24: 1–15
Barz W, Hüsemann W (1982) In: Fujiwara A (ed) Plant tissue culture 1982, Maruzen, Tokyo, pp 245–248
Berlyn MB, Zelitch I (1975) Plant Physiol. 56: 752–756
Eilert U, Nesbitt LR, Constable F (1985) Can. J. Bot. 63: 1540–1546
Farnsworth NR, Blomster RN, Damratoski D, Meer WA, Cammarato LV (1964) Lloydia 27: 302–314
Gamborg OL, Miller RA, Ojima K (1968) Exp. Cell Res. 50: 151–158
Hagimori M, Matsumoto T, Mikami Y (1984a) Plant Cell Physiol. 25: 947–953
Hagimori M, Matsumoto T, Mikami Y (1984b) Plant Cell Physiol. 25: 1099–1102
Horn ME, Dalton CC (1984) Int. Assoc. Plant Tissue Culture News letter 43: 2–7
Horn ME, Sherrard JH, Widholm JM (1983) Plant Physiol. 72: 426–429
Horn ME, Widholm JM (1984) In: Collins GB, Petolino JG (eds) Applications of genetic engineering to crop improvement, Nijhoff-Junk, Dordrecht, pp 113–161
Hüsemann W (1984) In: Vasil IK (ed) Cell culture and somatic cell genetics of plants, vol 1, Academic Press, Orlando, pp 182–191
Hüsemann W (1985) In: Vasil IK (ed) Cell culture and somatic cell genetics of plants, vol 2, Academic Press, Orlando, pp 213–252
Hüsemann W, Barz W (1977) Physiol. Plant. 40: 77–81
Jensen RG (1980) In: Stumpf PK, Conn EE (eds) The biochemistry of plants, vol 1, Academic Press, New York, pp 273–313
Kurz WGW, Chatson KB, Constabel F (1985) In: Neumann K-H, Barz W, Reinhard E (eds) Primary and secondary metabolism of plant cell cultures, Springer-Verlag, Berlin, pp 143–153
Kurz, WGW, Chatson KB, Constabel F, Kutney JP, Choi LSL, Kolodziejczyk P, Sleigh SK, Stuart KL, Worth BR (1980) Phytochem. 19: 2583–2587
Kurz WGW, Constabel F (1982) In: Wetter LR, Constabel F (eds) Plant tissue culture methods, National Research Council of Canada, Saskatoon, pp 128–131
Tyler RT (1984) J. Food Sci. 49: 925–930
Yamada Y, Sato F (1978) Plant Cell Physiol. 19: 691–699
Yamada Y, Sato F (1983) In: Evans DA, Sharp WR, Ammirato PV, Yamada Y (eds) Handbook of plant cell culture, vol 1, Macmillan Publishing Co., New York, pp 489–500
Yasuda T, Hashimoto T, Sato F, Yamada Y (1980) Plant Cell Physiol. 21: 929–932
Author information
Authors and Affiliations
Additional information
Communicated by F. Constabel
Rights and permissions
About this article
Cite this article
Tyler, R.T., Kurz, W.G.W. & Panchuk, B.D. Photoautotrophic cell suspension cultures of periwinkle (Catharanthus roseus (L.) G. Don): Transition from heterotrophic to photoautotrophic growth. Plant Cell Reports 5, 195–198 (1986). https://doi.org/10.1007/BF00269117
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00269117