Abstract
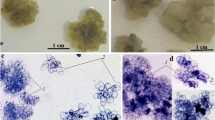
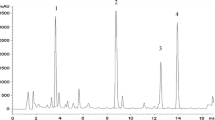
Plant cell and suspension cultures have been established from stem cuttings of Picrasma quassioides Bennett. The effect of 244 different types/concentrations of plant growth regulators on growth and quassin accumulation in callus tissue was investigated. Best growth, in terms of wet/dry weight after four weeks growth, was obtained on B5 media supplemented with 2% glucose, 10% coconut milk, 0.5 mg.l−1 zeatin riboside and 1.5 mg.l−1 IBA. The highest yields of quassin (0.014–0.018%) were detected on this same media supplemented with 1.0 mg.l−1 IBA and varying concentrations of zeatin riboside. Suspension cultures were easily established on B5 media supplemented with 2% glucose, 1.0 mg.l−1 2,4-D and 0.5 mg.l−1 kinetin. The carbon source had a marked effect on quassin accumulation with 0.32% quassin being detected when cells were grown in 2% galactose. This is comparable to the highest reported quassin yield for the whole plant.
Similar content being viewed by others
Abbreviations
- IAA:
-
indole-3-acetic acid
- IBA:
-
indolebutyric acid
- IpA:
-
N6-(Δ-isopentenyl) adenine
- IpAR:
-
N-(Δ-isopentenyl) adenine riboside
- NAA:
-
naphthalene acetic acid
- 2,4-D:
-
2,4-dichlorophenoxyacetic acid
- 6BA:
-
6-benzyladenine
References
Anderson L A, Harris A, Phillipson J D (1983) J Nat Prod 46: 374–378
Constabel F, Shyluk J P, Gamborg O L (1971) Planta 96: 306–316
Dix L, Van Staden J (1982) Plant Cell Tissue Organ Culture 1: 239–245
Furuya T, Kojima H, Syonok (1971) Phytochem 10: 1529–1532
Gamborg O L, Miller R A, Ojima K (1968) Expt. Cell Res 50: 151–158
Hikino H, Ohta T, Takemoto T (1975) Phytochem. 14: 2473–2481
London E, Robertson A, Worthington H (1950) J Chem Soc 3431
Murae T, Tsuyuki T, Ikeda T, Nishihama T, Masudo S, Takahashi T (1971) Tetrahedron: 27: 1545–1555 and 5147–5155.
Pierre A, Robert-Gero M, Tempete C, Polonsky J (1980) Biochem Biophys Res Comm 93: 675–680.
Polonsky J (1973a) Recent Advan Phytochem 6: 31–64
Polonsky J (1973b) Fortschr Chem Org Naturst 30: 101–150
Robins R J, Morgan M R A, Rhodes M J C, Furze J M (1984) Analytical Biochem 136: 145–156
Skoog F, Miller C O (1957) Symp Soc Exp Biol 11: 118–131
Author information
Authors and Affiliations
Additional information
Communicated by W. Barz
Rights and permissions
About this article
Cite this article
Scragg, A.H., Allan, E.J. Production of the triterpenoid quassin in callus and cell suspension cultures of Picrasma quassioides Bennett. Plant Cell Reports 5, 356–359 (1986). https://doi.org/10.1007/BF00268601
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00268601