Summary
We examined the intracellular localization of sugar residues of the rat gastric surface mucous cells in relation to the functional polarity of the cell organellae using preembedding method with several lectins.
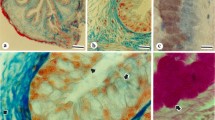
In the surface mucous cells, the nuclear envelope and rough endoplasmic reticulum (rER) and cis cisternae of the Golgi stacks were intensely stained with Maclura pomifera (MPA), which is specific to α-Gal and GalNAc residues. In the Golgi apparatus, one or two cis side cisternae were stained with MPA and Dolichos biflorus (DBA) which is specific to terminal α-N-acetylgalactosamine residues, while the intermediate lamellae were intensely labeled with Arachis hypogaea (PNA) which is specific to Galβ 1,3 GalNAc. Cisternae of the trans Golgi region were also stained with MPA, Ricinus communis I (RCA I) which is specific to β-Gal and Limax flavus (LFA) which is specific to α-NeuAc. Immature mucous granules which are contiguous with the trans Golgi lamellae were weakly stained with RCA I, while LFA stained both immature and mature granules.
The differences between each lectin's reactivity in the rough endoplasmic reticulum, in each compartment of the Golgi lamellae and in the secretory granules suggest that there are compositional and structural differences between the glycoconjugates in the respective cell organellae, reflecting the various processes of glycosylation in the gastric surface mucous cells.
Similar content being viewed by others
References
Baenziger JU, Fiete D (1979) Structural determinants of Ricinus communis agglutinin and toxin specificity for oligosaccharides. J Biol Chem 254:9795–9799
Baker DA, Sugii S, Kabat EA, Ratchiffe RM, Hermentin P, Lemieux RU (1983) Immunochemical studies on the combining sites of Forssman hapten reactive hemagglutinins from Dolichos biflorus, Helix pomatia, and Wistaria floribunda. Biochemistry 22:2741–2750
Bennett G, Leblond CP, Haddad A (1974) Migration of glycoprotein from the Golgi apparatus to the surface of various cell types as shown by radioautography after labeled fucose injection into rats. J Cell Biol 60:258–284
Bennett G, O'Shaughnessy D (1981) The site of incorporation of sialic acid residues into glycoproteins and the subsequent fates of these molecules in various rat and mouse cell types as shown by radioautography after injection of [3H] N-acetylmannosamine. I. Observations in Hepatocytes. J Cell Biol 88:1–15
Berger EG, Hesford FJ (1985) Localization of galactosyl- and sialyltransferase by immunofluorescence: evidence for different sites. Proc Natl Acad Sci USA 82:4736–4739
Bretton R, Bariety J (1976) A comparative ultrastructural localization of concanavalin A, wheat germ and Ricinus Communis on glomeruli of normal rat kidney. J Histochem Cytochem 10:1093–1100
Dawson PA, Filipe MI (1982) Changes in [3H] galactose uptake in human colonic mucosa with carcinoma: an ultrastructural study. Histochem J 14:361–383
Dunphy WG, Rothman JE (1983) Compartmentation of asparagine-linked oligosaccharide processing in the Golgi apparatus. J Cell Biol 97:270–275
Elhammer Å, Kornfeld S (1984) Two enzymes involved in the synthesis of O-linked oligosaccharides are localized on membranes of different densities in mouse lymphoma BW5147 cells. J Cell Biol 98:327–331
Etzler ME, Kabat EA (1970) Purification and characterization of a lectin (plant hemagglutinin) with blood group A specificity from Dolichos biflorus. Biochemistry 9:869–877
Fischer J, Klein PJ, Vierbuchen M, Skutta B, Uhlenbruck G, Fischer R (1984) Characterization of glycoconjugates of human gastrointestinal mucosa by lectins. I. Histochemical distribution of lectin binding sites in normal alimentary tract as well as in benign and malignant gastric neoplasms. J Histochem Cytochem 32:681–689
Fleischer B (1983) Mechanism of glycosylation in the Golgi apparatus. J Histochem Cytochem 31:1033–1040
Friend DS, Murry MJ (1965) Osmium impregnation of the Golgi apparatus. Am J Anat 117:135–150
Goldberg DE, Kornfeld S (1983) Evidence for extensive subcellular organization of asparagine-linked oligosaccharide processing and lysosomal enzyme phosphorylation. J Biol Chem 258:3159–3165
Goldstein IJ, Poretz RP (1986) Isolation, physicochemical characterization, and carbohydrate-binding specificity of lectins. In: Liener IE, Sharon N, Goldstein IJ (eds) The lectins — properties, functions, and applications in biology and medicine. Orlando, USA, pp 33–247
Griffiths G, Brands R, Burke B, Louvard D, Warren G (1982) Viral membrane proteins acquire galactose in trans Golgi cisternae during intracellular transport. J Cell Biol 95:781–792
Hanover JA, Lennarz WJ (1981) Transmembrane assembly of membrane and secretory glycoproteins. Arch Biochem Biophys 211:1–19
Hill HD Jr, Schwyzer M (1977) Ovine submaxillary mucin. Primary structure and peptide substrates of UDP-N-α-acetylgalactosamine: mucin transferase. J Biol Chem 252:3799–3804
Hubbard SC, Ivatt RJ (1981) Synthesis and processing of asparagine-linked oligosaccharides. Ann Rev Biochem 50:555–583
Ihida K, Suganuma T, Tsuyama S, Murata F (1988) Glycoconjugate histochemistry of the rat fundic gland using Griffonia simplicifolia agglutinin-II during the development. Am J Anat 182:250–256
Ihida K, Tsuyama S, Murata F (1990) The use of Griffonia simplicifolia agglutinin-IB4 in staining the developing rat fundic gland. Acta Histochem Cytochem 23:475–486
Isobe Y, Chen Shin-Tse, Nakane PK, Brown WR (1977) Studies on translocation of immunoglobulins across intestinal epithelium I. Improvements in the peroxidase-labeled antibody method for application to study of human intestinal mucosa. Acta Histochem Cytochem 10:161–171
Kim YS, Perdomo J, Nordberg J (1971) Glycoprotein biosynthesis in small intestinal mucosa. J Biol Chem 246:5466–5476
Kramer MF, Geuze JJ (1977) Glycoprotein transport in the surface mucous cells of the rat stomach. J Cell Biol 73:533–547
Kramer MF, Geuze JJ (1980) Comparison of various methods to localize a source of radioactivity in ultrastructural autoradiographs. The site of [3H]galactose incorporation in surface mucous cells of the rat stomach. J Histochem Cytochem 28:381–387
Letts PJ, Pinteric L, Schachter H (1974) Localization of glycoprotein glycosyltransferases in the Golgi apparatus of rat and mouse testis. Biochem Biophys Acta 372:304–320
Lotan R, Skutelsky E, Danon D, Sharon N (1975) The purification, composition, and specificity of the anti-T lectin from peanut (Arachis hypogaea). J Biol Chem 250:8518–8523
Miller RL, Collawn F Jr, Fish WW (1982) Purification and macromolecular properties of a sialic acid-specific lectin from the slug Limax flavus. J Biol Chem 257:7574–7580
Munro JR, Narasimhan S, Wetmore S, Riordan JR, Schachter H (1975) Intracellular localization of GDP-l-fucose: glycoprotein and CMP-sialic acid: apolipoprotein glycosyltransferases in rat and pork livers. Archs Biochem Biophys 169:269–277
Neutra M, Leblond CP (1966) Radioautographic comparison of the uptake of galactose-H3 and glucose-H3 in the Golgi region of various cells secretion glycoprotein or mucopolysaccharides. J Cell Biol 73:533–547
Nicolson GL (1974) The interactions of lectins with animal cell surfaces. Int Rev Cytol 39:90–190
Nicolson GL, Irimura T (1984) Estimating glycoprotein carbohydrate chain structures by lectin reactivities in polyacrylamide gels. Biol Cell 51:157–164
Novogrodsky A, Lotan R, Ravid A, Sharon N (1975) Peanut agglutinin, a new mitogen that binds to galactosyl sites exposed after neuraminidase treatment. J Immunol 115:1243–1248
Oliver C, Hand AR (1983) Enzyme modulation of the Golgi apparatus and GERL: a cytochemical study of parotid acinar cells. J Histochem Cytochem 31:1041–1048
Orci L, Vassalli J-D, Perrelet A (1988) The insulin factory. Sci Amer 259:50–61
Osawa T, Tsuji T (1987) Fractionation and structural assessment of oligosaccharides and glycopeptides by use of immobilized lectins. Ann Rev Biochem 56:21–42
Paulson JC, Rearick JI, Hill RL (1977) Enzymatic properties of β-d-galactoside α-2-6 sialyltransferase from bovine colostrum. J Biol Chem 252:2363–2371
Pavelka M, Ellinger A (1985) Localization of binding sites for concanavalin A, Ricinus communis I and Helix pomatia lectin in the Golgi apparatus of rat small intestinal absorptive cells. J Histochem Cytochem 33:905–914
Pavelka M, Ellinger A (1986) RCA I-binding patterns of the Golgi apparatus. Eur J Cell Biol 41:270–278
Pavelka M, Ellinger A (1987) The Golgi apparatus in the acinar cells of the developing embryonic pancreas: I. Morphology and enzyme cytochemistry. Am J Anat 178:215–223
Roth J (1983) Application of lectin-gold complexes for electron microscopic localization of glycoconjugates on thin sections. J Histochem Cytochem 31:987–999
Roth J (1984) Cytochemical localization of terminal N-acetyle-d-galactosamine residues in cellular compartments of intestinal goblet cells: implications for the topology of O-glycosylation. J Cell Biol 98:399–406
Roth J, Berger EG (1982) Immunocytochemical localization of galactosyltransferase in HeLa cells: codistribution with thiamine pyrophosphatase in trans-Golgi cisternae. J Cell Biol 92:223–229
Roth J, Lucocq JM, Charest PM (1984) Light and electron microscopic demonstration of sialic acid residues with the lectin from Limax flavus: a cytochemical affinity technique with the use of fetuin-gold complexes. J Histochem Cytochem 32:1167–1176
Sarkar M, Wu AM, Kabat EA (1981) Immunochemical studies on the carbohydrate specificity of Maclura pomifera lectin. Arch Biochem Biophys 209:204–218
Sato A, Spicer SS (1982a) Ultrastructural visualization of galactose in the glycoprotein of gastric surface cells with a peanut lectin conjugate. Histochem J 14:125–138
Sato A, Spicer SS (1982b) Ultrastructural visualization of galactosyl residues in various alimentary epithelial cells with the peanut lectin-horseradish peroxidase procedure. Histochemistry 73:607–624
Schulte BA, Spicer SS, Miller RL (1984) Histochemical localization of sialoglycoconjugates with sialic acid-specific lectin from the slug Limax flavus. Histochem J 16:1125–1132
Schwyzer M, Hill RL (1977) Procine a blood group-specific N-acetylgalactosaminyltransferase. J Biol Chem 252:2338–2345
Sharon N, Lis H (1972) Lectins: cell-agglutinating and sugar-specific proteins. Science 177:949–959
Skutelsky E, Lotan R, Sharon N, Danon D (1977) Distribution of the T-antigen on erythroid cell surfaces studies with peanut agglutinin, an anti-T specific lectin. Biochem Biophys Acta 467:165–174
Slot JW, Geuze HJ (1983) Immunoelectron microscopic exploration of the Golgi complex. J Histochem Cytochem 31:1049–1056
Sutoh K, Rosenfeld L, Lee YC (1977) Isolation of peanut lectin by affinity chromatography on polyacrylamide-entrapped guar beads and polyacrylamide (co-allyl α-d-galactopylanoside). Analyt Biochem 79:329–337
Strous GJAM (1979) Initial glycosylation of proteins with acetylgalactosaminylserine linkages. Proc Natl Acad Sci USA 76:2694–2698
Tartakoff AM, Vassalli P (1983) Lectin-binding sites as markers of Golgi subcompartments: Proximal-to-distal maturation of oligosaccharides. J Cell Biol 97:1243–1248
Tsuyama S, Suganuma T, Ihida K, Murata F (1986) Ultracytochemistry of glycosylation sites of rat colonic mucous cells with labeled lectins. Acta Histochem Cytochem 19:555–565
Yotsumoto S, Tsuyama S, Tashiro M, Murata F (1990) Ultracytochemistry of glycoconjugates in rat Brunner's gland with labeled lectins. J Electron Microsc 39:26–32
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ihida, K., Tsuyama, S., Kashio, N. et al. Subcompartment sugar residues of gastric surface mucous cells studied with labeled lectins. Histochemistry 95, 329–335 (1991). https://doi.org/10.1007/BF00266959
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00266959