Summary
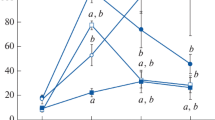
The localization of cytochrome P-450 of 17 α-hydroxylase/C17–C20 lyase (P-45017 α, lyase) and the changes of the enzyme activity were studied immunocytochemically and biochemically in the ovaries of immature rats treated with PMSG (pregnant mare serum gonadotropin) and hCG (human chorionic gonadotropin). Immunocytochemically, P-45017 α, lyase was localized in both the theca interna cells and interstitial gland cells of the ovaries of immature rats treated with PMSG for 48 h. After hCG administration, the immunoreactive cells rapidly decreased in number in the PMSG-pretreated rat ovary. Namely, 6 h after the hCG injection, positive staining for P-45017 α, lyase was recognized only in a few theca interna cells, while 12 h after the injection to immunostained cells were detected in the ovary. Forty-eight hours after the hGC treatment (96 h after the PMSG injection), most of the theca interna cells and the interstitial gland cells became immunopositive for P-45017 α, lyase again. The 17 α-hydroxylating activity of P-45017 α, lyase was 0.5, 0.22 and 0.03 nmol/min/mg protein in the ovarian microsomes of PMSG-treated, PMSG+hCG(3 h)-treated and PMSG+hCG(6 h)-treated rats, respectively. Changes of the hydroxylase activities in all the experimental groups are almost parallel to those of P-450 contents in the microsomes. These immunocytochemical and biochemical findings suggest that 1) P-45017 α, lyase is localized in both the theca interna cell and interstitial gland cell, and these cells are the main site of the androstenedione production in the ovary, and that 2) the decreased production of estrogen occurring just before ovulation is not brought about by the decreased activity of P-45017 α, lyase, but done by the loss of the enzyme.
Similar content being viewed by others
References
Bjersing L (1967) Histochemical demonstration Δ5-3 β- and 17 β-hydroxysteroid dehydrogenase activities in porcine ovary. Histochemie 10:295–304
Butcher RL, Collins WE, Fugo NW (1974) Plasma concentration of LH, FSH, prolactin, progesterone and estradiol-17 β throughout the 4-day estrous cycle of the rat. Endocrinology 94:1704–1708
Hedin L, Rodgers RJ, Simpson ER, Richards JS (1987) Changes in content of cytochrome P45017 α, cytochrome P450scc, and 3-hydroxy-3-methylglutaryl CoA reductase in developing rat ovarian follicles and corpora lutea: correlation with theca cell steroidogenesis. Biol Reprod 37:211–223
Ishimura K, Yoshinaga-Hirabayashi T, Tsuri H, Fujita H, Osawa Y (1989) Further immunocytochemical study on the localization of aromatase in the ovary of rats and mice. Histochemistry 90:413–416
Kominami S, Shinzawa K, Takemori S (1982) Purification and some properties of cytochrome P-450 specific for steroid 17 α-hydroxylation and C17-C20 bond cleavage from guinea pig adrenal microsomes. Biochem Biophys Res Commun 109:916–921
Kominami S, Inoue S, Higuchi A, Takemori S (1989) Steroidogenesis in liposomal system containing adrenal microsomal cytochrome P-450 electron transfer components. Biochim Biophys Acta 985:293–299
Le Goascogne C, Sananes N, Gouezou M, Baulieu EE, Robel P (1989) Cell-specific variations and hormonal regulation of immunoreactive cytochrome P-450scc in rat ovary. J Reprod Fertil 85:61–72
Lieberman S, Greenfield NJ, Wolfson A (1984) A heuristic proposal for understanding steroidogenic process. Endocr Rev 5:128–149
Omura T, Sato R (1964) The carbon monoxide-binding pigment of liver microsomes. I. Evidence for its hemoprotein nature. J Biol Chem 239:2370–2378
Pupkin M, Bratt H, Weisz J, Lloyd CW, Balogh K Jr (1966) Dehydrogenases in the rat ovary. I. A histochemical study of Δ5-3 β- and 20 α-hydroxysteroid dehydrogenases and enzymes of carbohydrate oxidation during the estrous cycle. Endocrinology 79:316–327
Rodgers RJ, Rodgers HF, Hall PF, Waterman MR, Simpson ER (1986) Immunolocalization of cholesterol side-chain-cleavage cytochrome P-450 and 17 α-hydroxylase cytochrome P-450 in bovine ovarian follicles. J Reprod Fertil 78:627–638
Sasano H, Okamoto M, Mason JI, Simpson ER, Mendelson CR, Sasano N, Silverberg SG (1989) Immunolocalization of aromatase, 17 α-hydroxylase and side-chain-cleavage cytochromes P-450 in the human ovary. J Reprod Fertil 85:163–169
Shinzawa K, Ishibashi S, Murakoshi M, Watanabe K, Kominami S, Kawahara A, Takemori S (1988) Relationship between zonal distribution of microsomal cytochrome P-450s (P-45017 α, lyase and P-450C 21) and steroidogenic activities in guinea-pig adrenal cortex. J Endocrinol 119:191–200
Suzuki K, Kawakura K, Tamaoki B (1978) Effect of pregnant mare's serum gonadotrophin on the activities of Δ4-5 α-reductase, aromatase, and other enzymes in the ovaries of immature rats. Endocrinology 102:1595–1605
Suzuki K, Tamaoki B (1979) Enzymological studies of rat luteinized ovaries in relation to acute reduction of aromatizable androgen formation and stimulated production of progestins. Endocrinology 104:1317–1323
Suzuki K, Tamaoki B (1983) Acute decrease by human chorionic gonadotropin of the activity of preovulatory ovarian 17 α-hydroxylase and C-17-C-20 lyase is due to decrease of microsomal cytochrome P-450 through de novo synthesis of ribonucleic acid and protein. Endocrinology 113:1985–1991
Yoshinaga-Hirabayashi T, Ishimura K, Fujita H, Inano H, Ishii-Ohba H, Tamaoki B (1987) Immunocytochemical localization of 17 β-hydroxysteroid dehydrogenase (17 β-HSD), and its relation to the ultrastructure of steroidogenic cells in immature and mature rat ovaries. Arch Histol Jpn 50:545–556
Yoshinaga-Hirabayashi T, Ishimura K, Fujita H, Kitawaki J, Osawa Y (1990) Immunocytochemical localization of aromatase in immature rat ovaries treated with PMSG and hCG, and in pregnant rat ovaries. Histochemistry 93:223–228
Zhong C, Ishimura K, Yoshinaga-Hirabayashi T, Fujita H, Kitawaki J, Osawa Y (1989) Immunocytochemical study on the localization of aromatase in the ovary of golden hamster, guinea pig and cow. Acta Histochem Cytochem 22:501–507
Zlotkin T, Farkash Y, Orly J (1986) Cell-specific expression of immunoreactive cholesterol side-chain cleavage cytochrome P-450 during follicular development in the rat ovary. Endocrinology 119:2809–2820
Author information
Authors and Affiliations
Additional information
Supported by grants from the Ministry of Education, Science and Culture, Japan
Rights and permissions
About this article
Cite this article
Ishimura, K., Yoshinaga-Hirabayashi, T., Tsuri, H. et al. Immunocytochemical and biochemical studies on the localization and changes of 17 α-hydroxylase/C17–C20 lyase activity in immature rat ovary treated with PMSG and hCG. Histochemistry 94, 225–229 (1990). https://doi.org/10.1007/BF00266622
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00266622