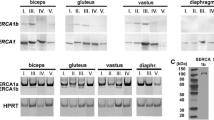
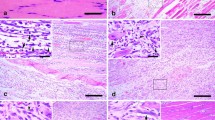
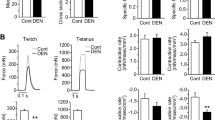
Summary
In the present study we have investigated the reactivity of rat muscle to a specific monoclonal antibody directed against alpha cardiac myosin heavy chain. Serial cross sections of rat hindlimb muscles from the 17th day in utero to adulthood, and after neonatal denervation and de-efferentation, were studied by light microscope immunohistochemistry. Staining with anti-α myosin heavy chain was restricted to intrafusal bag fibres in all specimens studied. Nuclear bag2 fibres were moderately to strongly stained in the intracapsular portion and gradually lost their reactivity towards the ends, whereas nuclear bag1 fibres were stained for a short distance in each pole. Nuclear bag2 fibres displayed reactivity to anti-α myosin heavy chain from the 21st day of gestation, whereas nuclear bag1 fibres only acquired reactivity to anti-α myosin heavy chain three days after birth. After neonatal de-efferentation, the reactivity of nuclear bag2 fibres to anti-α myosin heavy chain was decreased and limited to a shorter portion of the fibre, whereas nuclear bag1 fibres were unreactive. We showed that a myosin heavy chain isoform hitherto unknown for skeletal muscle is specifically expressed in rat nuclear bag fibres. These findings add further complexity to the intricate pattern of isomyosin expression in intrafusal fibres. Furthermore, we show that motor innervation influences the expression of this isomyosin along the length of the fibres.
Similar content being viewed by others
References
Barker D, Banks RW (1986) The muscle spindle. In: Engel AG, Banker BQ (eds) Myology, McGraw-Hill, New York, pp 309–341
Buller AJ, Eccles JC, Eccles RM (1960) Interactions between motor neurones and muscles in respect of the characteristic speeds of their responses. J Physiol 150:417–439
Butler-Browne G, Whalen RG (1984) Myosin isozyme transitions occuring during the postnatal development of the rat soleus muscle. Dev Biol 102:324–334
Chizzonite RA, Zak R (1984) Regulation of myosin isoenzyme composition in fetal and neonatal rat ventricle by endogenous thyroid hormones. J Biol Chem 259:12628–12632
Condon K, Silberstein L, Blau HM, Thompson W (1990) Differentiation of fibre types in aneural musculature of the prenatal rat hindlimb. Dev Biol 138:275–295
Dubowitz V, Brooke MH (1973) Muscle biopsy: a modern approach. In: Major problems in neurology. WB Saunders, London
Eriksson PO, Butler-Browne GS, Fischman DA, Grove BK, Schiaffino S, Virtanen I, Thornell L-E (1988) Myofibrillar and cytoskeletal proteins in human muscle spindles. In: Hník P, Soukup T, Vejsada R, Zelená J (eds) Mechanoreceptors — development, structure and function. Plenum Press, New York, pp 273–274
Harris AJ (1981) Embryonic growth and innervation of rat skeletal muscles. Philos Trans R Soc Lond [Biol] 293:257–277
Hoh JFY, Hughes S (1988) Myogenic and neurogenic regulation of myosin gene expression in cat jaw closing muscles regenerating in fast and slow muscle beds. J Muscle Res Cell Motil 9:59–72
Hoh JFY, Hughes S, Hugh G, Pozgaj I (1989) Three hierarchies in skeletal muscle fibres classification: allotype, isotype and phenotype. In: Stockdale FE, Kedes LH (eds) UCLA Symposia on Molecular and Cellular Biology, vol 93. Liss, New York, pp 15–26
Kennedy JM, Kamel S, Tambone WW, Vrbova G, Zak R (1986) The expression of myosin heavy chain isoforms in normal and hypertrophied chicken slow muscle. J Cell Biol 103:977–983
Kilby K, Dhoot GK (1988) Identification and distribution of some developmental isoforms of myosin heavy chains in avian muscle fibres. J Muscle Res Cell Motil 9:516–524
Kronnie G te, Donselaar Y, Soukup T, van Raamsdonk W (1981) Histochemical differences in myosin composition among intrafusal muscle fibres. Histochemistry 73:65–74
Kronnie G te, Donselaar Y, Soukup T, Zelená J (1982) Development of immunohistochemical characteristics of intrafusal fibres in normal and defferented rat muscle spindles. Histochemistry 74:355–366
Kucera J, Donovini-Zis K, Engel WK (1978) Histochemistry of rat intrafusal muscle fibres and their motor innervation. J Histochem Cytochem 26:973–988
Kucera J, Walro JM, Reichler J (1989) Role of nerve and muscle factors in the development of rat muscle spindles. Am J Anat 186:144–160
Kucera J, Walro JM (1987) Postnatal maturation of spindles in deafferented rat soleus muscles. Anat Embryol 176:449–461
Kucera J, Walro JM (1988) The effect of neonatal deafferentation or deefferentation on myosin heavy chain expression in intrafusal muscle fibres of the rat. Histochemistry 90:151–160
Kucera J, Walro JM (1989) Nonuniform expression of myosin heavy chain isoforms along the length of cat intrafusal fibres. Histochemistry 92:291–299
Kucera J, Walro J (1990a) Myosin heavy chain expression in developing rat intrafusal muscle fibres. Neurosci Lett 109:18–22
Kucera J, Walro JM (1990b) Origin of intrafusal muscle fibres in the rat. Histochemistry 93:567–580
Leger JOC, Bouvagnet P, Pau B, Roncucci R, Leger JJ (1985) Levels of ventricular myosin fragments in human sera after myocardial infarction determined with monoclonal antibodies to myosin heavy chains. Eur J Clin Invest 15:422–429
Mahdavi V, Izumo S, Nadal-Ginard B (1987) Developmental and hormonal regulation of sarcomeric myosin heavy chain gene family. Circ Res 60:804–814
Maier A, Gambke B, Pette D (1988) Immunohistochemical demonstration of embryonic myosin heavy chains in adult mammalian intrafusal fibres. Histochemistry 88:267–271
McLennan IS (1983) Neural dependence and independence of myotube production in chicken hindlimb muscles. Dev Biol 98:287–294
Ovalle WK, Smith RS (1972) Histochemical identification of three types of intrafusal muscle fibres in the cat and monkey based on the myosin ATPase reaction. Can J Physiol Pharmacol 50:195–202
Pavlath GK, Rich K, Webster SG, Blau HM (1989) Localization of muscle gene products in nuclear domains. Nature 337:570–573
Pedrosa F, Butler-Browne GS, Dhoot GK, Fischman DA, Thornell L-E (1989) Diversity in expression of myosin heavy chain isoforms and M band proteins in rat muscle spindles. Histochemistry 92:185–194
Pedrosa F, Thornell L-E (1990) Expression of myosin heavy chain isoforms in developing rat muscle spindles. Histochemistry 94:231–244
Pierobon-Bormioli S, Sartore S, Vitadello M, Schiaffino S (1980) “Slow” myosins in vertebrate skeletal muscle. J Cell Biol 85:672–681
Ross JJ, Duxon MJ, Harris AJ (1987) Neural determination of muscle fibre numbers in embyronic rat lumbrical muscle. Development 100:395–409
Rowlerson A, Gorza L, Schiaffino S (1985) Immunohistochemical identification of spindle fibre types in mammalian muscle using type-specific antibodies to isoforms of myosin. In: Boyd IA, Gladden MH (eds) The muscle spindle. McMillan, London, pp 29–34
Sawchak J, Leung B, Shafiq SA (1985) Characterization of a monoclonal antibody to myosin specific for mammalian and human type 2 muscle fibres. J Neurol Sci 69:247–254
Schiaffino S, Gorza L, Sartore S, Saggin L, Ansoni S, Vianello M, Gundersen K, Lømo T (1989) Three myosin heavy chain isoforms in type 2 skeletal muscle fibers. J Muscle Res Cell Motil 10:197–205
Soukup T (1976) Intrafusal fibre types in rat limb muscle spindles. Histochemistry 47:43–57
Soukup T, Pedrosa F, Thornell L-E (1990) Influence of neonatal motor denervation on expression of myosin heavy chain isoforms in rat muscle spindles. Histochemistry 94:245–256
Stockdale FE, Miller JB (1987) The cellular basis of myosin heavy chain isoform expression during development of avian skeletal muscle. Dev Biol 123:1–9
Stockdale FE, Miller JB, Feldman JL, Lamson G, Hager J (1989) Myogenic cell lineages: commitment and modulation during differentiation of avian muscle. In: Stockdale FE, Kedes LH (eds) UCLA Symposia on Molecular and Cellular Biology, vol 93. Liss, New York, pp 3–13
Termin A, Staron RS, Pette D (1989) Myosin heavy chain isoforms in histochemically defined fiber types of rat muscle. Histochemistry 92:453–457
Thornell L-E, Eriksson PO, Fischman DA, Grove BK, Butler-Browne GS, Virtanen I (1988) Human muscle spindle development. In: Zelená J, Hník P (eds) Mechanoreceptors: development, structure and function. Plenum Press, New York, pp 39–44
Thornell L-E, Grove BK, Pedrosa F, Butler-Browne GS, Dhoot GK, Fischman DA (1989) Expression of slow tonic myosin in muscle spindle fibres early in mammalian development. Cellular and molecular biology of muscle development. In: Stockdale FE, Kedes LH (eds) UCLA Symposia on Molecular and Cellular Biology, vol 93. Liss, New York, pp 471–480
Whalen RG (1985) Myosin isoenzymes as molecular markers for muscle physiology. J Exp Biol 115:43–53
Zelená J (1957) The morphogenetic influence of innervation on the ontogenic development of mechanoreceptors. J Embryol Exp Morphol 5:283–292
Zelená J (1976) The role of sensory innervation in the development of mechanoreceptors. Prog Brain Res 43:69–74
Zelená J, Soukup T (1973) Development of muscle spindles deprived of fusimotor innervation. Z Zellforsch 144:435–452
Zelená J, Soukup T (1974) The differentiation of intrafusal fibre types in rat muscle spindles after motor denervation. Cell Tissue Res 153:115–136
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pedrosa, F., Soukup, T. & Thornell, L.E. Expression of an alpha cardiac-like myosin heavy chain in muscle spindle fibres. Histochemistry 95, 105–113 (1990). https://doi.org/10.1007/BF00266582
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00266582