Summary
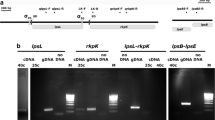
DNA fragments carrying the recA genes of Rhizobium meliloti and Rhizobium leguminosarum biovar viciae were isolated by complementing a UV-sensitive recA − Escherichia coli strain. Sequence analysis revealed that the coding region of the R. meliloti recA gene consists of 1044 by coding for 348 amino acids whereas the coding region of the R. leguminosarum bv. viciae recA gene has 1053 bp specifying 351 amino acids. The R. meliloti and R. leguminosarum bv. viciae recA genes show 84.8% homology at the DNA sequence level and of 90.1% at the amino acid sequence level. recA − mutant strains of both Rhizobium species were constructed by inserting a gentamicin resistance cassette into the respective recA gene. The resulting recA mutants exhibited an increased sensitivity to UV irradiation, were impaired in their ability to perform homologous recombination and showed a slightly reduced growth rate when compared with the respective wild-type strains. The Rhizobium recA strains did not have altered symbiotic nitrogen fixation capacity. Therefore, they represent ideal candidates for release experiments with impaired strains.
Similar content being viewed by others
References
Akaboshi E, Yip MLR, Howard-Flanders P (1989) Nucleotide sequence of the recA gene of Proteus mirabilis. Nucleic Acids Res 17:4390
Arnold W, Pühler A (1988) A family of high copy number plasmid vectors with single end-label sites for rapid nucleotide sequencing. Gene 70:171–179
Ball TK, Wasmuth CR, Braunagel SC, Benedik MJ (1990) Expression of Serratia marcescens extracellular proteins requires recA. J Bacteriol 172:342–349
Benedict RC, Kowalczykowski SC (1988) Increase of the DNA strand assimilation activity of RecA protein by removal of the C terminus and structure-function studies of the resulting protein fragment. J Biol Chem 263:15513–15520
Beringer JE (1974) R factor transfer in Rhizobium leguminosarum. J Gen Microbiol 84:188–198
Better M, Helinski DR (1983) Isolation and characterization of the recA gene of Rhizobium meliloti. J Bacteriol 155:311–316
Boyer HW, Roulland-Dussoix D (1969) A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol 41:459–472
De Vos GF, Walker GC, Signer ER (1986) Genetic manipulations in Rhizobium meliloti utilizing two new transposon Tn5 derivatives. Mol Gen Genet 204:485–491
Farrand SK, O'Morchoe SP, McCutchan J (1989) Construction of an Agrobacterium tumefaciens C58 recA mutant. J Bacteriol 171:5314–5321
Gill DR, Hatfull GH, Salmond GPC (1986) A new cell division operon in Escherichia coli. Mol Gen Genet 201:134–145
Hickman MJ, Orser CS, Willis DK, Lindow SE, Panopoulos NJ (1987) Molecular cloning and biological characterization of the recA gene from Pseudomonas syringae. J Bacteriol 169:2906–2910
Hynes MF, Simon R, Müller P, Niehaus K, Labes M, Pühler A (1986) The two megaplasmids of Rhizobium meliloti are involved in the effective nodulation of alfalfa. Mol Gen Genet 202:356–362
Hynes MF, Bruksch K, Priefer U (1988) Melanin production encoded by a cryptic plasmid in a Rhizobium leguminosarum strain. Arch Microbiol 150:326–332
Kawashima H, Horii T, Ogawa T, Ogawa H (1984) Functional domains of Escherichia coli RecA protein deduced from the mutational sites in the gene. Mol Gen Genet 193:288–292
Keener SL, McNamee KP, McEntee K (1984) Cloning and characterization of recA genes from Proteus vulgaris, Erwinia carotovora, Shigella fexneri and Escherichia coli B/r. J Bacteriol 160:153–160
Klapwijk P, van Belen P, Schilperoort R (1979) Isolation of a recombination deficient Agrobacterium tumefaciens mutant. Mol Gen Genet 173:171–175
Knight KL, McEntee K (1986) Nucleotide binding by a 24-residue peptide from the recA protein of Escherichia coli. Proc Natl Acad Sci USA 83:9289–9293
Knight KL, Hess RM, McEntee K (1988) Conservation of an ATP-binding domain among recA proteins from Proteus vulgaris, Erwinia carotovora, Shigella fexneri and Escherichia coli K-12 and B/r. J Bacteriol 170:2427–2432
Larminat F, Defais M (1989) Modulation of the SOS response by truncated RecA proteins. Mol Gen Genet 216:106–112
Lipman DJ, Pearson WR (1985) Rapid and sensitive protein similarity searches. Science 227:1435–1441
Little JW (1984) Autodigestion of LexA and phage repressors. Proc Natl Acad Sci USA 81:1375–1379
Maniatis R, Fritsch EF, Sambrook J (1982) Molecular cloning. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Maxam AM, Gilbert W (1977) A new method for sequencing DNA. Proc Natl Acad Sci USA 74:560–564
Miles CA, Mountain A, Sastre GRK (1986) Cloning and characterization of the recA gene of Agrobacterium tumefaciens C58. Mol Gen Genet 204:161–165
Miller JH (1972) Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Murphy RC, Gasparich GE, Bryant DA, Porter RD (1990) Nucleotide sequence and further characterization of the Synechococcus sp. strain PCC 7002 recA gene: Complementation of a cyanobacterial recA mutation by the Escherichia coli recA gene. J Bacteriol 172:967–976
Nakazawa T, Kimoto M, Abe M (1990) Cloning, sequencing, and transcriptional analysis of the recA gene of Pseudomonas cepacia. Gene 94:83–88
Ogawa H, Ogawa T (1986) General recombination: Functions and structure of recA protein. Adv Biophys 21:135–148
Priefer U (1989) Genes involved in lipopolysaccharide production and symbiosis are clustered on the chromosome of Rhizobium leguminosarum biovar viciae VF39. J Bacteriol 171:6161–6168
Priefer UB, Simon R, Pühler A (1985) Extension of the host range of Escherichia coli vectors by incorporation of RSF1010 replication and mobilization functions. J Bacteriol 163:324–330
Putney SD, Melendez DLD, Schimmel PR (1981) Cloning, partial sequencing, and in vitro transcription of the gene for alanine tRNA synthetase. J Biol Chem 256:205–211
Ried JL, Collmer A (1987) An npt-sacB-sacR cartridge for constructing directed, unmarked mutations in Gram-negative bacteria by marker exchange-eviction mutagenesis. Gene 57:239–246
Sancar A, Stachelek C, Konigsberg W, Rupp WD (1980) Sequences of the recA gene and protein. Proc Natl Acad Sci USA 77:5463–5467
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain-terminating inhibitors. Proc Nat1 Acad Sci USA 74:5463–5467
Sano Y, Kageyama M (1987) The sequence and function of the recA gene and its protein in Pseudomonas aeruginosa PAO. Mol Gen Genet 208:412–419
Simon R, Priefer U, Pühler A (1983) A broad host range mobilization system for in vivo genetic engineering: Transposon mutagenesis in Gram-negative bacteria. Biotechnology 1:748–751
Slilaty SN, Little JW (1987) Lysine-156 and serine-119 are required for LexA repressor cleveage: A possible mechanism. Proc Natl Acad Sci USA 84:3987–3991
Smith GR (1988) Homologous recombination in prokaryotes. Microbiol Rev 52:1–28
Turner GL, Gibson AH (1980) Measurement of nitrogen fixation by indirect means. In: Methods for evaluating biological nitrogen fixation. Bergersen FJ (ed) John Wiley & Sons, Chichester, pp 111–138
Vieira J, Messing J (1982) The pUC plasmids, a M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene 19:259–268
Vincent JM (1970) A manual for the practical study of root nodule bacteria. (IBP handbook no 15) Blackwell, Oxford
Walker GC (1984) Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol Rev 48:60–93
Wang WB, Tessman ES (1986) Location of functional regions of the Escherichia coli RecA protein by DNA sequence analysis of RecA protease-constitutive mutants. J Bacteriol 168:901–910
Zhao XJ, McEntee K (1990) DNA sequence analysis of the recA genes from Proteus vulgaris, Erwinia carotovora, Shigella flexneri and Escherichia coli B/r. Mol Gen Genet 222:369–376
Author information
Authors and Affiliations
Additional information
Communicated by J. Schell
The accession numbers: X59956 R. LEGUMINOSARUM REC A ALAS-DNA; X59957 R. MELITOTI REC A ALAS-DNA
Rights and permissions
About this article
Cite this article
Selbitschka, W., Arnold, W., Priefer, U.B. et al. Characterization of recA genes and recA mutants of Rhizobium meliloti and Rhizobium leguminosarum biovar viciae . Molec. Gen. Genet. 229, 86–95 (1991). https://doi.org/10.1007/BF00264217
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00264217