Abstract
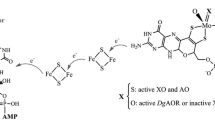
The tungsten- and the molybdenum-containing aldehyde oxidoreductases from Clostridium formicoaceticum show, for aldehydes, K m values<30 μM and K i values of millimolar concentrations. The tungsten-containing aldehyde oxidoreductase is inactivated to 50% by 3 mM KCN within 1 min, by 1 mM ferricyanide within 5 min, and by 0.05 mM chloralhydrate within 30 s. The molybdenum-containing AOR shows 50% inactivation within 1 min only with 70 mM KCN. The tungsten-containing enzyme is very sensitive to oxygen, especially in the reduced state, whereas the molybdenum-containing enzyme exhibits only moderate oxygen sensitivity without being markedly influenced by the redox state of the enzyme. The tungsten in the aldehyde oxidoreductase is bound to a pterin cofactor (Wco) of the mononucleotide form that is known for molybdopterin cofactor (Moco). The nature of the molybdenum cofactor in the molybdenum-containing aldehyde oxidoreductase is still unclear. The UV/VIS spectrum of the tungsten-containing aldehyde oxidoreductase shows a broad absorption in the range of 400 nm with a millimolar absorption coefficient of 18.1 (reduced form) and 24.8 (dehydrogenated form) at 396 nm. The epr spectrum exhibits two different W(V) signals with the following g values for signal A: 2.035, 1.959, 1.899 and signal B: 2.028, 2.017, 2.002. Dithionite-reduced enzyme shows signals of 4Fe−4S or 2Fe−2S clusters. Initial rate studies with different substrates for the carboxylate reduction led to a Bi Uni Uni Bi mechanism.
Similar content being viewed by others
Abbreviations
- AOR :
-
aldehyde oxidoreductase
- NH 2 CO-MV :
-
1,1′-carbamoylmethylviologen
- MV :
-
methylviologen
- TMV :
-
1,1′,2,2′-tetramethylviologen
References
Barata BAS, LeGall J, Moura JJG (1993) Aldehyde oxidoreductase activity in Desulfovibrio gigas: in vitro reconstitution of an electron transfer chain from aldehydes to the production of molecular hydrogen. Biochemistry 32:11559–11568
Bergmeyer HU, Graßl M, Walter HE (1983) Methods of enzymatic analysis, 3rd edn, vol II. Verlag Chemie, Weinheim, p 144
Bray RC (1975) Molybdenum iron-sulfur flavin hydroxylases and related enzymes. In: Boyer PD (ed) The enzymes, vol XII, 3rd edn. Academic Press, New York, pp 300–417
Cleland WW (1963) The kinetics of enzyme-catalysed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim Biophys Acta 67:104–137
Coughlan MP (1980) Molybdenum and molybdenum-containing enzymes. Pergamon Press, Oxford, pp 119–185
Deaton JC, Solomon EI, Watt GD, Wetherbee PJ, Durfor CN (1987) Electron paramagnetic resonance studies of the tungsten-containing formate dehydrogenase from Clostridium thermoaceticum. Biochem Biophys Res Commun 149:424–430
Durfor CN, Wetherbee PJ, Deaton JC, Solomon EI (1983) Characterization and spectroscopic properties of reduced Mo and W formate dehydrogenase from C. thermoaceticum. Biochem Biophys Res Commun 115:61–67
Fetzner S, Lingens F (1993) Purification and some properties of the molybdenum- and iron-containing quinaldic acid 4-oxidoreductase from Serratia marcescens 2CC-1. Biol Chem Hoppe-Seyler 374:363–376
Gardlik S, Rajagopalan KV (1991) Oxidation of molybdopterin in sulfite oxidase by ferricyanide. J Biol Chem 266:4889–4895
Hille R (1992) Xanthine oxidase, xanthine dehydrogenase, and aldehyde oxidase. J Biochem Flavoenzymes 3:21–68
Johnson JL, Rajagopalan KV, Mukund S, Adams MWW (1993) Identification of molybdopterin as the organic component of the tungsten cofactor in four enzymes from hyperthermophilic archaea. J Biol Chem 268:4848–4852
Krüger B, Meyer O, (1986) The pterin (bactopterin) of carbon monoxide dehydrogenase from Pseudomonas carboxydoflava. Eur J Biochem 157:121–128
Krüger B, Meyer O, Nagel M, Andreesen JR, Meincke M, Bock E, Blümle S, Zumft WG (1987) Evidence for the presence of bactopterin in the eubacterial molybdoenzymes nicotinic acid dehydrogenase, nitrite oxidoreductase, and respiratory nitrate reductase. FEMS Microbiol Lett 48:225–227
Leonhardt U, Andreesen JR (1977) Some properties of formate dehydrogenase, accumulation and incorporation of 185W-tungsten into proteins of Clostridium formicoaceticum. Arch Microbiol 115:277–284
Mukund S, Adams MWW (1991) The novel tungsten-iron-sulfur protein of the hyperthermophilic archaebacterium, Pyrococcus furiosus, is an aldehyde ferredoxin oxidoreductase. J Biol Chem 266:14208–14216
Mukund S, Adams MWW (1993) Characterization of a novel tungsten-containing formaldehyde ferredoxin oxidoreductase from the hyperthermophilic archaeon, Thermococcus litoralis. J Biol Chem 268:13592–13600
Romao MJ, Barata BAS, Archer M, Lobeck K, Moura I, Carrondo MA, Legall J, Lottspeich F, Huber R, Moura JJG (1993) Subunit composition, crystallization and preliminary cristallographic studies of the Desulfovibrio gigas aldehyde oxidoreductase containing molybdenum and [2Fe−2S] centers. Eur J Biochem 215:729–732
Rudolph FB, Fromm HJ (1983) Plotting methods for analyzing enzyme rate data. In: Purich DL (ed) Contemporary enzyme kinetics and mechanism. Academic Press, New York, pp 53–73
Schmitz RA, Albracht SPJ, Thauer RK (1992a) A molybdenum and a tungsten isoenzyme of formylmethanofuran dehydrogenase in the thermophilic archaeon Methanobacterium wolfei. Eur J Biochem 209:1013–1018
Schmitz RA, Albracht SPJ, Thauer RK (1992b) Properties of the tungsten-substituted molybdenum formylmethanofuran dehydrogenase from Methanobacterium wolfei. FEBS Lett 309:78–81
Schmitz RA, Richter M, Linder D, Thauer RK (1992c) A tungsten-containing active formylmethanofuran dehydrogenase in the thermophilic archaeon Methanobacterium wolfei. Eur J Biochem 207:559–565
Segel JH (1975) Enzyme kinetics. Wiley, New York, pp 665–749
Strobl G, Feicht R, White H, Lottspeich F, Simon H (1992) The tungsten-containing aldehyde oxidoreductase from Clostridium thermoaceticum and its complex with a viologen accepting NADPH oxidoreductase. Biol Chem Hoppe-Seyler 373:123–132
Thanos I, Bader J, Günther H, Neumann S, Krauss F, Simon H (1987) Electroenzymatic and electromicrobial reduction: preparation of chiral compounds. Methods Enzymol 136:302–317
White H, Simon H (1992) The role of tungstate and/or molybdate in the formation of aldehyde oxidoreductase in Clostridium formicoaceticum and other acetogens. Arch Microbiol 158:81–84
White H, Strobl G, Feicht R, Simon H (1989) Carboxylic acid reductase: a new tungsten enzyme catalyses the reduction of nonactivated carboxylic acids to aldehydes. Eur J Biochem 184:89–96
White H, Feicht R, Huber C, Lottspeich F, Simon H (1991) Purification and some properties of the tungsten-containing carboxylic acid reductase from Clostridium formicoaceticum. Biol Chem Hoppe-Seyler 372:999–1005
White H, Huber C, Feicht R, Simon H (1993) On a reversible molybdenum-containing aldehyde oxidoreductase from Clostridium formicoaceticum. Arch Microbiol 159:244–249
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Huber, C., Caldeira, J., Jongejan, J.A. et al. Further characterization of two different, reversible aldehyde oxidoreductases from Clostridium formicoaceticum, one containing tungsten and the other molybdenum. Arch. Microbiol. 162, 303–309 (1994). https://doi.org/10.1007/BF00263776
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00263776