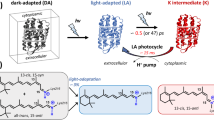
Abstract
The chromophore in halorhodopsin (HR) which acts as a light-driven chloride pump in halobacteria shares many properties with its counterpart in bacteriorhodopsin (BR): (i) a similar retinal protein interaction, (ii) trans to cis isomerization and (iii) similar intermediates of its photocycle. One major difference between the two chromoproteins is that the HR chromophore does not become deprotonated during its photocycle. A mechanism for the photocycle of HR is presented, which, in close analogy to an earlier proposed mechanism for BR, involves the sequence of all-trans → 13-cis, 14s-cis → 13-cis → all-trans isomerizations of the chromophore, a Schiff base of retinal. In contrast to the situation in BR the 13-cis, 14s-cis→13-cis isomerization is induced not by deprotonation of the retinal Schiff base chromophore but rather by the movement of an anion (Cl-) towards the protonated nitrogen of the Schiff's base. The suggested mechanism involves the Schiff base directly in the chloride translocation in halorhodopsin.
Similar content being viewed by others
References
Alshuth T, Stockburger M, Hegemann P, Oesterhelt D (1985) Structure of the retinal chromophore in halorhodopsin. FEBS Lett 179:55–59
Ariki M, Schobert B, Lanyi J (1986) Effects of arginine modification on the halorhodopsin photocycle. Biophys J 49: 476a
Aton B, Doukas AG, Callender RH, Becher B, Ebrey TG (1977) Resonance Raman studies of the purple membrane. Biochemistry 16:2995–2999
Bamberg E, Hegemann P, Oesterhelt D (1984a) The chromoprotein of halorhodopsin is the light-driven electrogenic chloride pump in Halobacterium halobium. Biochemistry 23:6216–6221
Bamberg E, Hegemann P, Oesterhelt D (1984b) Reconstitution of the light-driven electrogenic ion pump halorhodopsin in black lipid membranes. Biochim Biophys Acta 773: 53–60
Bogomolni RA, Belliveau, JW, Weber HJ (1981) Salt dependent spectral transition of Halobacterium pigment P588. Biophys J 33:217a (Abstr. T-pm-f4)
Braiman M, Mathies R (1980) Resonance Raman evidence for an all-trans to 13-cis isomerization in the proton-pumping cycle of bacteriorhodopsin. Biochemistry 19:5421–5428
Brock CJ, Tanner MJA, Kempf C (1983) The human erythrocyte anion transport protein. Partial amino acid sequence conformation and a possible molecular mechanism. Biochem J 213:577–586
Falke JJ, Chan SI, Steiner M, Oesterhelt D, Towner P, Lanyi JK (1984) Halide binding by the purified halorhodopsin chromoprotein. II. New chloride binding sites revealed by 35-Cl NMR. J Biol Chem 259:2185–2189
Gärtner W, Towner P, Hopf H, Oesterhelt D (1983) Removal of methyl groups from retinal and its influence on the function of bacteriorhodopsin. Biochemistry 22:2637–2644
Gerwert K, Siebert F (1986) Evidence for a light-induced 13-cis, 14s-cis isomerization in bacteriorhodopsin obtained by FITR difference spectroscopy using isotopically labelled retinals. EMBO J 5:805–811
Gödecke M-E (1976) Identifizierung der bei der Umsetzung von Bakteriorhodopsin mit Hydroxylamin erhaltenen Produkte. Diplomarbeit, Würzburg
Harbison GS, Smith SO, Pardoen JA, Courtin JML, Lugtenburg J, Herzfeld J, Mathies RA, Griffin RG (1985) Solid state 13C-NMR detection of a perturbed 6-s-trans chromophore in bacteriorhodopsin. Biochemistry 24:6955–6962
Hazemoto N, Kamo N, Kobatake Y, Tsuda M, Terayama Y (1984) Effect of salt on photocycle and ion-pumping of halorhodopsin and third rhodopsinlike pigment of Halobacterium halobium. Biophys J 45:1073–1077
Hegemann P (1984) Halorhodopsin, die lichtgetriebene Chloridpumpe in Halobacterium halobium. Dissertation, Universität München
Hegemann P, Oesterhelt D, Steiner M (1985) The photocycle of the chloride pump halorhodopsin. I. Azide catalyzed deprotonation of the chromophore is a side reaction of photocycle intermediates inactivating the pump. EMBO J 4:2347–2350
Honig B, Greenberg AD, Dinur U, Ebrey TG (1976) Visulapigment spectra: implications of the protonation of the retinal Schiff base. Biochemistry 15:4993–4999
Lanyi JK (1984) Light-dependent trans to cis isomerization in halorhodopsin. FEBS Lett 175:337–342
Lanyi JK, Oesterhelt D (1982) Identification of the retinal-binding protein in halorhodopsin. J Biol Chem 257: 2674–2677
Lanyi JK, Vodyanoy V (1986) Flash spectroscopic studies of the kinetics of the halorhodopsin photocycle. Biochemistry 25:1465–1470
Oesterhelt D, Meentzen M, Schuhmann L (1973) Reversible dissociation of the purple complex in bacteriorhodopsin and identification of 13-cis and all-trans retinal as its chromophores. Eur J Biochem 40:453–463
Oesterhelt D, Hegemann P, Tittor J (1985) The photocycle of the chloride pump halorhodopsin. II. Quantum yields and a kinetic model. EMBO J 4:2351–2356
Ogurusu T, Maeda A, Sasaki N, Yoshizawa T (1982) Effects of chloride on the absorption spectrum and photoreactions of halorhodopsin. Biochim Biophys Acta 682:446–451
Ogurusu T, Maeda A, Sasaki N, Yoshizawa T (1981) Light-induced reaction of halorhodopsin prepared under low salt conditions. J Biochem (Tokyo) 90:1267–1273
Orlandi G, Schulten K (1979) Coupling of stereochemical and proton donor-acceptor properties of Schiff base. A model of light-driven proton pump. Chem Phys Lett 64:370–374
Polland H-J (1984) Die ersten Schritte der Photosynthese in den retinalhaltigen Proteinen Bakteriorhodopsin und Halorhodopsin. Dissertation, Technische Universität München
Polland H-J, Franz MA, Zinth W, Kaiser W, Hegemann P, Oesterhelt D (1985) Picosecond events in the photochemical cycle of the light-driven chloride-pump halorhodopsin. Biophys J 47:55–59
Schobert B, Lanyi JK (1982) Halorhodopsin is a light-driven chloride pump. J Biol Chem 257:10306–10313
Schobert B, Lanyi JK, Oesterhelt D (1986) Effects of anion binding on the deprotonation reactions of halorhodopsin. J Biol Chem 261:2690–2696
Schulten K, Tavan P (1978) A mechanism for the light-driven proton pump of Halobacterium halobium. Nature 272: 85–86
Schulten K, Schulten Z, Tavan P (1984) An isomerization model for the pump cycle of bacteriorhodopsin. In: Bolis L (ed). Information and energy transduction in biological membranes. Alan R Liss, New York, pp 113–131
Sheves M, Baasov T (1984) Factors affecting the rate of thermal-isomerization of 13-cis-bacteriorhodopsin to all-trans. J Am Chem Soc 106:6840–6841
Smith SO, Marvin MJ, Bogomolni RA, Mathies, RA (1984) Structure of the retinal chromophore in the HR578 form of halorhodopsin. J Biol Chem 259:12326–12329
Smith SO, Lugtenburg J, Mathies R (1985) Determination of retinal chromophore structure in bacteriorhodopsin with Resonance Raman spectroscopy. J Memb Biol 85:95–109
Spudich JL, McCain DA, Nakanishi K Okabe M, Shimizu N, Rodman H, Honig B, Bogomolni RA (1986) Chromophore-protein interaction in bacterial sensory rhodopsin and bacteriorhodopsin. Biophys J 49:479–483
Steiner M, Oesterhelt D (1983) Isolation and properties of the native chromoprotein halorhodopsin. EMBO J 2:1379–1385
Steiner M, Oesterhelt D, Ariki M, Lanyi JK (1983) Halide binding by the purified halorhodopsin chromoprotein. I. Effects on the chromophore. J Biol Chem 259:2179–2184
Tavan P, Schulten K (1986) Evidence for a 13,14-cis cycle in bacteriorhodopsin. Biophys J (in press)
Tavan P, Schulten K, Oesterhelt D (1985a) The effect of protonation and electrical interactions on the stereochemistry of retinal Schiff bases. Biophys J 47:415–430
Tavan P, Schulten K, Gärtner W, Oesterhelt D (1985b) Substituents at the C-13 position of retinal and their influence on the function of bacteriorhodopsin. Biophys J 47: 349–355
Thiel W (1981) The MNDOC method, a correlated version of the MNDO model. J Am Chem Soc 103:1413–1419
Weber HJ, Bogomolni RA (1981) A second retinal-containing pigment in Halobacterium halobium. Photochem Photobiol 33:601–608
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Oesterhelt, D., Hegemann, P., Tavan, P. et al. Trans-cis isomerization of retinal and a mechanism for ion translocation in halorhodopsin. Eur Biophys J 14, 123–129 (1986). https://doi.org/10.1007/BF00263069
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00263069