Abstract
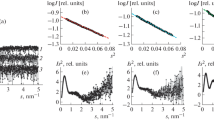
The quaternary structure of ribulose-1,5-bisphosphate carboxylase-oxygenase (rubisco) from Rhodospirillum rubrum, an enzyme consisting of two large subunits, L2, was investigated by small-angle X-ray scattering. In the presence of HCO -3 and Mg2+, rubisco is in the active state and displays a radius of gyration of 2.96 nm, a maximum diameter of 9.5 nm and a volume of 170 nm3. A model is presented where the subunits are arranged back-to-back, rotated relative to each other by 90°, and shifted by 1.3 nm. Upon inactivation by removal of HCO -3 and Mg2+, the model swells slightly without any distinct changes in configuration. This contrasts with our previous observations with rubisco from Alcaligenes eutrophus, an enzyme composed of small (S) and large (L) subunits, L8S8, where inactivation gives rise to substantial changes in configuration.
Similar content being viewed by others
Abbreviations
- RuBP:
-
Ribulose-1,5-bisphosphate
- 3-PGA:
-
3-phosphoglyceric acid
References
Bowien B, Gottschalk E-M (1982) Influence of the activation state on the sedimentation properties of ribulose bisphosphate carboxylase from Alcaligenes eutrophus. J Biol Chem 257:11845–11847
Bowien B, Mayer F, Codd GA, Schlegel HG (1976) Purification, some properties and quaternary structure of the d-ribulose 1,5-bisphosphate carboxylase of Alcaligenes eutrophus. Arch Microbiol 110:157–166
Bowien B, Mayer F, Spiess E, Pähler A, Englich U, Saenger W (1980) On the structure of crystalline ribulosebisphosphate carboxylase from Alcaligenes eutrophus. Eur J Biochem 106:4405–4410
Glatter O (1977) A new method for the evaluation of small-angle scattering data. J Appl Cryst 10:415–421
Glatter O (1980) Computation of distance distribution functions and scattering functions of models for small-angle scattering experiments. Acta Phys Austr 52:243–256
Glatter O, Kratky O (eds) (1982) Small-angle X-ray scattering. Academic Press, London New York
Meisenberger O, Pilz I, Bowien B, Pal GP, Saenger W (1984) Small-angle X-ray study on the structure of active and inactive ribulose bisphosphate carboxylase from Alcaligenes eutrophus. J Biol Chem 259:4463–4465
Porod G (1951) Die Röntgenkleinwinkelstreuung von dichtgepackten kolloiden Systemen. Kolloid-Z 124:83–114
Schloss JV, Phares EF, Long MV, Norton IL, Stringer CD, Hartman FC (1979) Isolation, characterization, and crystallization of ribulosebisphosphate carboxylase from autotrophically grown Rhodospirillum rubrum. J Bacteriol 137:490–501
Tabita FR, McFadden BA (1974) d-Ribulose 1,5-bisphosphate carboxylase from Rhodospirillum rubrum. II. Quaternary structure, composition, catalytic, and immunological properties. J Biol Chem 249:3459–3464
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wilhelm, P., Abuja, P.M., Meisenberger, O. et al. Small-angle X-ray study on the structure of ribulose-1,5-bisphosphate carboxylase-oxygenase from Rhodospirillum rubrum . Eur Biophys J 14, 93–96 (1986). https://doi.org/10.1007/BF00263065
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00263065