Summary
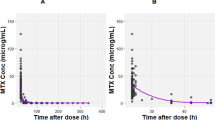
The absorption, distribution, and elimination kinetics of low-dose p.o. methotrexate (MTX) were repeatedly studied in 19 children during maintenance treatment of childhood acute lymphoblastic leukemia. Plasma concentrations, urinary elimination, and bone marrow concentrations of MTX and 7-hydroxymethotrexate (7-OH-MTX) were monitored during 24 h following a routime p.o. dose (30 mg/m2) using high-pressure liquid chromatography. Significant interindividual variability was found in time to peak concentration (30–180 min), peak concentration (0.41–2.77 μM), and to a lesser extent the half-lives (t1/2α: 32.8–86.1 min; t1/2β: 43.6–350.0 min; t1/2 absorption: 25.2–60.3 min) and plasma area under the concentration-time curve from zero to infinity (195.6–818.5 μM.min). Significant amounts of 7-OH-MTX were detected in plasma, with a mean area under the concentration-time curve from zero to infinity of 208 μM.min compared with 365.6 μM.min for MTX. High concentrations of 7-OH-MTX were present in bone marrow 24 h after oral MTX (15/19 patients) and were at least five fold those in plasma and three fold the concentration of MTX in bone marrow. In four patients occasionally neither MTX nor metabolite could be detected. Repeated examination of these pharmacokinetic parameters in plasma and bone marrow showed that the intraindividual variability was small.
Similar content being viewed by others
References
Aherne GW, Quinton M (1981) Techniques for the measurement of methotrexate in biological samples. Cancer Treat Rep [Suppl 1] 65: 55
Balis FM, Savitch JL, Bleyer WA (1983) Pharmacokinetics of oral methotrexate in children. Cancer Res 43: 2342
Canfell C, Sadée W (1980) Methotrexate and 7-hydroxymethotrexate: serum level monitoring by high performance liquid chromatography. Cancer Treat Rep 64: 165
Chan KK, Balachandran Nayar MS, Cohen JL, Chlebowsky RT, Liebman H, Stolinsky D, Farquhar D (1980) Metabolism of methotrexate in man after high and conventional doses. Res Commun Chem Pathol Pharmacol 28: 551
Craft AW, Rankin A, Aherne W (1981) Methotrexate absorption in children with acute lymphoblastic leukaemia. Cancer Treat Rep [Suppl 1] 65: 77
Erttman R, Bielack S, Landbeck G (1985) Kinetics of 7-hydroxymethotrexate after high dose methotrexate therapy. Cancer Chemother Pharmacol 15: 101
Evans WE, Hutson PR, Stewart CF (1983) Methotrexate cerebrospinal fluid and serum concentrations after intermediatedose methotrexate infusions. Clin Pharmacol Ther 33: 301
Evans WE, Stewart CF, Chen CH, Cran WR, Bowman WP, Abromowitch M, Simone JV (1984) Methotrexate systemic clearance influences probability of relapse in children with standard-risk acute lymphocytic leukaemia. Lancet 1: 359
Fabre G, Matherly LH, Fabre I, Cano JP, Goldman ID (1984) Interactions between 7-hydroxymethotrexate and methotrexate at the cellular level in the Ehrlich Ascites tumor in vitro. Cancer Res 44: 970
Farquhar D, Loo TL, Vadlamudi S (1972) Synthesis and biological evaluation of 7-hydroxymethotrexate, 7-methylaminopterin and 7-methylmethotrexate. J Med Chem 15: 567
Howell SK, Wang YM, Hosoya R, Sutow WW (1980) Plasma methotrexate as determined by liquid chromatography, enzyme-inhibition assay and radio-immunoassay after high dose infusion. Clin Chem 26: 734
Jacobs SA, Stoller RG, Chabner BA, Johns DG (1976) 7-Hydroxymethotrexate as a urinary metabolite in human subjects and rhesus monkeys receiving high-dose methotrexate. J Clin Invest 57: 534
Jacobs SA, Stoller RG, Chabner BA, Johns DG (1977) Dosedependent metabolism of methotrexate in man and rhesus monkeys. Cancer Treat Rep 61: 651
Kamen BA, Holcenberg JS, Turo K, Whitehead VM (1983) Variability of red blood cell folate and methotrexate in children with acute lymphocytic leukemia. Proc. Am Assoc Cancer Res 135 (abstract)
Kearney PJ, Light PA, Preece A, Mott MG (1979) Unpredictable serum levels after oral methotrexate in children with acute lymphoblastic leukaemia. Cancer Chemother Pharmacol 3: 117
Lankelma J, Poppe H (1978) Determination of methotrexate in plasma by on-column concentration and ion-exchange chromatography. J Chromatogr 149: 587
Lankelma J, van der Klein E (1980) The role of 7-hydroxymethotrexate during methotrexate anti-cancer therapy. Cancer Lett 9: 133
Pearson ADJ, Craft AW, Eastham EJ, Aherne GW, Littleton P, Pearson GL, Campbell AN (1985) Small intestinal transit time affects methotrexate absorption in children with acute lymphoblastic leukemia. Cancer Chemother Pharmacol 14: 211
Pinkerton CR, Welshman SG, Dempsey SI, Bridges JM, Glasgow JFT (1980) Absorption of methotrexate under standardised conditions in children with acute lymphoblastic leukaemia. Br J Cancer 42: 613
Pinkerton CR, Glasgow JFT, Bridges JM, Welshman SG (1981) Enterotoxic effect of methotrexate: does it influence the drug's absorption in children with acute lymphoblastic leukaemia? Br Med J 282: 1276
Piper JR, McCaleb GS, Montgomery JA, Kisliuk RL, Gaumant Y, Sirotnah FM (1982) 10-Propargylaminopterin and alkyl homologues of methotrexate as inhibitors of folate metabolism. J Med Chem 25: 877
Stam AJ, Van der Kogel AJ, Nooter K (1985) Effect of X-irradiation on the pharmacokinetics of methotrexate in rats: alteration of the blood-brain barrier. Eur J Cancer Clin Oncol 21: 759
Stewart AL, Margison JM, Wilkinson PM, Lucas SB (1985) The pharmacokinetics of 7-hydroxymethotrexate following medium-dose methotrexate therapy. Cancer Chemother Pharmacol 14: 165
Wagner J (1975) Fundamentals of clinical pharmacokinetics. Hamilton, Ill.
Wang YM, Howell SK, Smith RG, Hosoya K, Benvenuto JA (1979) Effect of metabolism on pharmacokinetics and toxicity of high-dose methotrexate therapy in children. Proc Am Soc Clin Oncol 20: 334
Author information
Authors and Affiliations
Additional information
This study was supported by the Netherlands Cancer Foundation “Koningin Wilhelmina Fonds”
Rights and permissions
About this article
Cite this article
Sonneveld, P., Schultz, F.W., Nooter, K. et al. Pharmacokinetics of methotrexate and 7-hydroxy-methotrexate in plasma and bone marrow of children receiving low-dose oral methotrexate. Cancer Chemother. Pharmacol. 18, 111–116 (1986). https://doi.org/10.1007/BF00262278
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00262278