Abstract
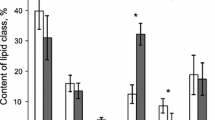
In order to investigate nutritional interactions in the symbiotic scleractinian coral-zooxanthella association, fatty acids of the coral Galaxea fascicularis were analysed in two groups of cultured microcolonies. The first group was fed with Artemia sp., while the second group was starved. After an initial 1-month period during which both groups were subjected to the same “normal” light conditions (constant irradiance of 125 μE·cm-2·s-1 and 14:10 h light:dark), a light cap was used to cover the aquarium and keep all the microcolonies in permanent darkness for 20 days. During the light phase of the experiment it was shown that the nutritional status lead to large variations in the percentage of saturated, mono-unsaturated and polyunsaturated fatty acids. Palmitic acid (C16:0) was the most abundant fatty acid in both groups. Important differences between fed and starved microcolonies occurred during the dark phase of the experiment. In the fed group the dark phase was characterized by a significant increase in polyunsaturated fatty acids. Particularly arachidonic acid (C20:4 n-6) became the most important fatty acid followed by docosatrienoic acid (C22:3 n-3). A slight increase in these two fatty acids was also found in the starved group but the bulk of polyunsaturated fatty acids was significantly decreased. In this group, palmitic acid remained the most important fatty acid while an increased concentration of cis-vaccenic acid (C18:1 n-7) was found at the end of the experiment. The increased concentration of cis-vaccenic acid might indicate that bacteria serve as a source of energy. While the number of zooxanthellae per milligram of protein and the chlorophyll a to protein ratio strongly decreased in the starved microcolonies immediately after the beginning of the dark period, the decrease in fed microcolonies was delayed for about 10 days. Furthermore, after 20 days of dark incubation the chlorophyll a to protein ratio was the same as measured at the beginning of the dark period. This suggests that in the dark the metabolic requirements of the zooxanthellae are in part met from the animal host through a heterotrophic mode of nutrition.
Similar content being viewed by others
Abbreviations
- CZ :
-
cultured zooxanthellae
- FAME :
-
fatty acid methylester(s)
- FDM :
-
fed dark microcolonies
- FLM :
-
fed light microcolonies
- MUFA :
-
monounsaturated fatty acid(s)
- PUFA :
-
polyunsaturated fatty acid(s)
- SDM :
-
starved dark microcolonies
- SFA :
-
saturated fatty acids
- SLM :
-
starved-light microcolonies
- SW :
-
sea water
- TFA :
-
total fatty acids
References
Al-Moghrabi S, Allemand D, Jaubert J (1993) Valine uptake by the scleractinian coral Galaxea fascicularis: characterization and effect of light and nutritional status. J Comp Physiol B 163: 355–362
Baar HJW de, Farrington JW, Wakeham SG (1983) Vertical flux of fatty acids in the north Atlantic Ocean. J Mar Res 41: 9–41
Bell MV, Henderson RJ, Sargent JR (1986) The role of polyunsaturated fatty acids in fish. Comp Biochem Physiol 83B: 711–719
Ben-Mlih F, Marty J-C, Fiala-Médioni A (1992) Fatty acid composition in deep hydrothermal vent symbiotic bivalves. J Lipid Res 33: 1797–1806
Benson AA, Muscatine L (1974) Wax in coral mucus: energy transfer from corals to reef fishes. Limnol Oceanogr 19: 810–814
Bishop DC, Kenrick JR (1980) Fatty acid composition of symbiotic zooxanthellae in relation to their hosts. Lipid 15: 799–804
Blank RJ (1987) Cell architecture of the dinoflagellate Symbiodinium sp. inhabiting the Hawaiian stony coral Montipora verrucosa. Mar Biol 94: 143–155
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37: 911–917
Clayton WS, Lasker HR (1984) Host feeding regime and zooxanthellal photosynthesis in the anemone, Aiptasia pallida (Verrill). Biol Bull 167: 590–600
Conway N, McDowell Capuzzo J (1991) Incorporation and utilization of bacterial lipids in the Solemya velum symbiosis. Mar Biol 108: 277–291
Crossland CJ, Barnes DJ, Borowitzka MA (1980) Dirunal lipid and mucus production in the staghorn coral Acropora acuminata. Mar Biol 60: 81–90
Erez J (1990) On the importance of food sources in coral-reef ecosystems. In: Dubinsky Z (ed) Coral reefs, ecosystems of the world 25. Elsevier, Amsterdam, pp 411–418
Falkowski PG, Dubinsky Z (1981) Light-shade adaptation of Stylophora pistillata, a hermatypic coral from the Gulf of Eilat. Nature 289: 172–174
Farrant PA, Borowitzka MA, Hinde R, King RJ (1987) Nutrition of the temperate Australian soft coral Capnella gaboensis. II. The role of zooxanthellae and feeding. Mar Biol 95: 575–581
Fitt WK, Pardy RL (1981) Effects of starvation, and light and dark on the energy metabolism of symbiotic and aposymbiotic sea anemones, Anthopleura elegantissima. Mar Biol 61: 199–205
Fitt WK, Pardy RL, Littler MM (1982) Photosynthesis, respiration, and contribution to community productivity of the symbiotic sea anemone Anthopleura elegantissima (Brandt 1835). J Exp Mar Biol Ecol 61: 213–232
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol chem 226: 497–509
Gillan FT, Johns RB (1986) Chemical markers for marine bacteria: fatty acids and pigments. In: Johns RB (ed) Biological markers in the sedimentary environment. Elsevier, Amsterdam, pp 291–309
Hama T (1991) Production and turnover rates of fatty acids in marine particulate matter through phytoplankton photosynthesis. Mar Chem 33: 213–227
Harland AD, Davies PS, Fixter LM (1992a) Lipid content of some Caribbean corals in relation to depth and light. Mar Biol 113: 357–361
Harland AD, Fixter LM, Davies PS, Anderson RA (1991) Distribution of lipids between the zooxanthellae and animal compartment in the symbiotic sea anemone Anemonia viridis: wax esters, triglycerides and fatty acids. Mar Biol 110: 13–19
Harland AD, Fixter LM, Davies PS, Anderson RA (1992b). Effect of light on the total lipid content and storage lipids of the symbiotic sea anemone Anemonia viridis. Mar Biol 112: 253–258
Harland AD, Navarro JC, Davies PS, Fixter LM (1993) Lipids of some Caribbean and Red Sea corals: total lipids, wax esters, triglycerides and fatty acids. Mar Biol 117: 113–117
Jeffrey SW, Humphrey GF (1975) New spectrophotometric equations for determining chlorophylls a, b, c 1 and c 2 in higher plants, algae and natural phytoplankton. Biochem Physiol Pflanzen 167: 191–194
Johannes RE (1974) Sources of nutritional energy for reef corals. Proc 2nd Int Coral Reef Symp 1: 133–137
Joint IR, Morris RJ (1982) The role of bacteria in the turnover of organic matter in the sea. Oceanogr Mar Biol Annu Rev 20: 65–118
Kevin KM, Hudson RCL (1979) The role of zooxanthellae in the hermatypic coral Plesiastrea urvillei (Milne Edwards and Haime) from cold waters. J Exp Mar Biol Ecol 36: 157–170
Latyshev NA, Naumenko NV, Svetashev VI, Latipov YY (1991) Fatty acids of reef-building corals. Mar Ecol Prog Ser 76: 295–301
Léger P, Bengtson DA, Simpson KL, Sorgeloos P (1986) The use and nutritional value of Artemia as a food source. Oceanogr Mar Biol Annu Rev 24: 521–623
Loeblich III AR (1984) Dinoflagellate physiology and biochemistry. In: Spector DL (ed) Dinoflagellates. Academic Press, New York, pp 299–342
Lovern JA (1964) The lipids of marine organisms. Oceanogr Mar Biol Annu Rev 2: 169–191
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193: 265–275
McCloskey LR, Muscatine L (1984) Production and respiration in the Red Sea coral Stylophora pistillata as a function of depth. Proc R Soc Lond B 222: 215–230
Metcalfe LD, Schmitz AA (1961) The rapid preparation of fatty acid esters for gas chromatographic analysis. Anal Chem 33: 363–364
Meyers PA (1977) Fatty acids and hydrocarbons of Caribbean corals. Proc 3rd Int Coral Reef Symp 1: 529–536
Meyers PA (1979) Polyunsaturated fatty acids in coral: indicators of nutritional sources. Mar Biol Letters 1: 69–75
Meyers PA, Porter JW, Chard RL (1978) Depth analysis of fatty acids of two Caribbean reef corals. Mar Biol, 49: 197–202
Miller DJ, Yellowlees D (1989) Inorganic nitrogen uptake by symbiotic marine cnidarians: a critical review. Proc R Soc Lond B 237: 109–125
Muller-Parker G (1984) Photosynthesis-irradiance responses and photosynthetic periodicity in the sea anemone Aiptasia pulchella and its zooxanthellae. Mar Biol 82: 225–232
Muller-Parker G (1985) Effect of feeding regime and irradiance on the photophysiology of the symbiotic sea anemone Aiptasia pulchella. Mar Biol 90: 65–74
Muscatine L (1973) Nutrition of corals. In: Jones OA, Endean R (eds) Biology and geology of coral reefs. II Biology 1. Academic Pres, New York, pp 77–112
Muscatine L (1990) The role of symbiotic algae in carbon and energy flux in reef corals. In: Dubinsky Z (ed) Coral Reefs, ecosystems of the World 25, Elsevier, Amsterdam, pp 75–87
Muscatine L, McCloskey LR, Marian ER (1981) Estimating the daily contribution of carbon from zooxanthellae to coral animal respiration. Limnol Oceanogr 26: 601–611
Muscatine L, Weis V (1992) Productivity of zooxanthellae and biogeochemical cycles. In: Falkowski PG, Woodhead AD (eds) Primary productivity and biogeochemical cycles in the sea. Plenum Press, New York, pp 257–271
Patton JS, Abraham S, Benson AA (1977) Lipogenesis in the intact coral Pocillopora capitata and its isolated zooxanthellae: evidence for a light-driven carbon cycle between symbiont and host. Mar Biol 44: 235–247
Piorreck M, Baasch K-H, Pohl P (1984) Biomass production, total protein, chlorophylls, lipids and fatty acids of fresh water green and blue-green algae under different nitrogen regimes. Phytochemistry 23: 207–216
Porter JW, Muscatine L, Dubinsky Z, Falkowski PG (1984) Primary production and photoadaptation on light and shade adapted colonies of the symbiotic coral Stylophora pistillata. Proc R Soc Lond B 222: 161–180
Radwan SS, Shaaban AS, Gebreel HM (1988) Arachidonic acid in the lipids of marine algae maintained under blue, white and red light. Z Naturforsch 43C: 15–18
Rasmussen C (1988) Effects of nutrients carried by mainland runoff on reefs of the Cairns area: a research plan and preliminary results. In: Baldwin CL (ed) Nutrients in the Great Barrier Reef region. Great Barrier Reef Marine Park Authority Workshop Series No. 10, pp 66–91
Risk MJ, Sammarco PW (1991) Cross-shelf trends in skeletal density of the massive coral Porites lobata from the Great Barrier Reef. Mar Ecol Prog Ser 69: 195–200
Sargent JR (1976) The structure, metabolism and function of lipids in marine organisms. In: Malins DC, Sargent JR (eds) Biochemical and biophysical perspectives in marine biology, vol. 3. Academic Press, New York, pp 149–212
Sargent J, Bell MV, Henderson RJ, Tocher DR (1990) Polyunsaturated fatty acids in marine and terrestrial food webs. In: Mallinger J (ed) Animal nutrition and transport process. 1. Nutrition in wild and domestic animals. Karger, Basel, pp 11–23
Sargent JR, Parkes RJ, Mueller-Harvey I, Henderson RJ (1987) Lipid biomarkers in marine ecology. In: Sleigh MA (ed) Microbes in the sea. Ellis Horwood, Chichester, pp 119–138
Sargent JR, Whittle KJ (1981) Lipids and hydrocarbons in the marine food web. In: Longhurst AR (ed) Analysis of marine ecosystems. Academic Press, Toronto, pp 491–533
Scribe P, Fillaux J, Laureillard J, Denant V, Saliot A (1991) Fatty acids as biomarkers of planktonic inputs in the stratified estuary of the Krka River, Adriatic Sea: relationship with pigments. Mar Chem 32: 299–312
Schlichter D (1980) Adaptation of cnidarians for integumentary absorption of dissolved organic material. Rev Can Biol 39: 259–282
Sorokin YI (1973) On the feeding of some scleractinian corals with bacteria and dissolved organic matter. Limnol Oceanogr 18: 380–385
Sorokin YI (1981) Aspects of the biomass, feeding and metabolism of common corals of the great barrier reef, Australia. Proc 4th Int Coral Reef Symp 2: 27–32
Stanley-Samuelson DW (1987) Physiological roles of prostaglandines and other eicosanoids in invertebrates. Biol Bull 173: 92–109
Steen RG (1986) Evidence for heterotrophy by zooxanthellae in symbiosis with Aiptasia pulchella. Biol Bull 170: 267–278
Steen RG (1987) Evidence for facultative heterotrophy in cultured zooxanthellae. Mar Biol 95: 15–23
Steen RG, Muscatine L (1984) Daily budgets of photosynthetically fixed carbon in symbiotic Zoanthids. Biol Bull 167: 477–487
Steen RG, Muscatine L (1987) Low temperature evokes rapid exocytosis of symbiotic algae by a sea anemone. Biol Bull 172: 246–263
Stimson JS (1987) Location, quantity and rate of change in quantity of lipids in tissue of Hawaiin hermatypic corals. Bull Mar Sci 41: 889–904
Sukenik A, Wahnon R (1991) Biochemical quality of marine unicellular algae with special emphasis on lipid composition. I. Isochrysis galbana. Aquaculture 97: 61–72
Szmant-Froelich A, Pilson MEQ (1980) The effects of feeding frequency and symbiosis with zooxanthellae on the biochemical composition of Astrangia danae Milne Edwards and Haime. J Exp Mar Biol Ecol 48: 85–97
Viso A-C, Marty J-C (1993), Fatty acids from 28 marine microalgae. Phytochem 34: 1521–1533
Wakeham SG, Canuel EA (1988) Organic geochemistry of particulate matter in the eastern tropical north pacific ocean: Implication for particle dynamics. J Mar Res 46: 183–213
Author information
Authors and Affiliations
Additional information
Communicated by H. Huddart
Rights and permissions
About this article
Cite this article
Al-Moghrabi, S., Allemand, D., Couret, J.M. et al. Fatty acids of the scleractinian coral Galaxea fascicularis: effect of light and feeding. J Comp Physiol B 165, 183–192 (1995). https://doi.org/10.1007/BF00260809
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00260809