Summary
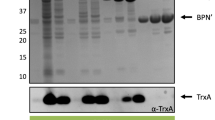
During an investigation into the substrate specificity and processing of subtilisin Carlsberg fromBacillus licheniformis, two major independent findings were made: (i) as has been shown previously, a stretch of five amino acids (residues 97–101 of the mature enzyme) that loops out into the binding cleft is involved in substrate binding by subtilisin Carlsberg. In order to see whether this loop element also determines substrate specificity, the coding region for these five amino acids was deleted from the cloned gene for subtilisin Carlsberg by site-directed mutagenesis. Unexpectedly the resulting mutant preproenzyme (P42c, Mr=42 kDa) was not processed to the mature form (Mr=30 kDa) and was not released into the medium by a proteasedeficientB. subtilis host strain; rather, it accumulated in the cell membrane. This result demonstrates that the integrity of this loop element, which is very distant from the processing cleavage sites in the preproenzyme, is required for secretion of subtilisin Carlsberg. (ii) In culture supernatants fromB. subtilis harbouring the cloned wild-type subtilisin Carlsberg gene the transient appearance (at 0–3 h after onset of stationary phase) of a processing intermediate (P38c, Mr=38 kDa) of this protease could be demonstrated. P38c very probably represents a genuine proform of subtilisin Carlsberg.
Similar content being viewed by others
References
Berger H, Goebel W, Kreft J, Bartnik F (1987) Alkalische Protease, Verfahren zur Herstellung von Hybridvektoren und genetisch transformierte Mikroorganismen. German patent DE 3527913A1
Bernhard K, Schrempf H, Goebel W (1978) Bacteriocin nd antibiotic resistance plasmids inBacillus cereus andBacillus subtilis. J Bacteriol 133:897–903
Bode W, Papamokos E, Musil D (1987) The high-resolution X-ray crystal structure of the complex formed between subtilisin Carlsberg and eglin c, an elastase inhibitor from the leechHirudo medicinalis. Eur J Biochem 166:673–692
Chang S, Cohen SN (1979) High frequency transformation ofBacillus subtilis protoplasts by plasmid DNA. Mol Gen Genet 168:111–115
Gryczan TJ, Contente S, Dubnau D (1978) Characterization ofStaphylococcus aureus plasmids introduced by transformation intoBacillus subtilis. J Bacteriol 134:316–329
Ikeumura H, Inouye M (1988) In vitro processing of pro-subtilisin produced inEscherichia coli. J Biol Chem 263:12959–12963
Jacobs M, Eliasson M, Uhlen M, Flock JI (1985) Cloning, sequencing and expression of subtilisin Carlsberg fromBacillus licheniformis. Nucleic Acids Res 13:8913–8926
Kawamura F, Doi RH (1984) Construction of aBacillus subtilis double mutant defective in extracellular alkaline and neutral proteases. J Bacteriol 160:442–444
Koide Y, Nakamura A, Uozumi T, Beppu T (1986) Cloning and sequencing of the major intracellular serine protease gene ofBacillus subtilis. J Bacteriol 167:110–116
Kreft J, Berger H, Härtlein M, Müller B, Weidinger G, Goebel W (1983) Cloning and expression inEscherichia coli andBacillus subtilis of the hemolysin (cereolysin) determinant fromBacillus cereus. J Bacteriol 155:681–689
Kyhse-Andersen J (1984) Electroblotting of multiple gels: a simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. J Biochem Biophys Methods 10:203–209
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of the bacteriophage T4. Nature 227:680–685
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Markland FS, Smith EL (1971) Subtilisins: Primary structure, chemical and physical properties. In: Boyer PD (ed) The enzymes, vol III. Academic Press, New York, pp 561–608
Notermans S, Chakraborty T, Leimeister-Wächter M, Dufrenne J, Heuvelmann KJ, Maas H, Jansen W, Wernars K, Guinee P (1989) Specific gene probe for detection of biotyped and serotypedListeria strains. Appl Environ Microbiol 55:902–906
Osborn MJ, Gander JE, Parisi E, Carson J (1972) Mechanism of assembly of the outer membrane ofSalmonella typhimurium. J Biol Chem 247:3962–3972
Park SS, Wong SL, Wang LF, Doi RH (1989) TheBacillus subtilis subtilisin gene (aprE) is expressed from a σA (σ43) promoter in vitro and in vivo. J Bacteriol 171:2657–2665
Power SD, Adams RM, Wells JA (1986) Secretion and autoproteolytic maturation of subtilisin. Proc Natl Acad Sci USA 83:3096–3100
Russell AJ, Thomas PG, Fersht AR (1987) Electrostatic effects on modification of charged groups in the active site cleft of subtilisin by protein engineering. J Mol Biol 193:803–813
Sanger F, Nicklen S, Coulson HR (1977) DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74:5463–5467
Smith EL, DeLange RJ, Evans WH, Landon M, Markland FS (1968) Subtilisin Carlsberg V. The complete sequence; comparison with subtilisin BPN′; evolutionary relationships. J Biol Chem 243:2184–2191
Stahl ML, Ferrari E (1984) Replacement of theBacillus subtilis subtilisin structural gene with an in vitro-derived deletion mutation. J Bacteriol 158:411–418
Stanssens P, Opsomer C, McKeown YM, Kramer W, Zabeau M, Fritz HJ (1989) Efficient oligonucleotide-directed construction of mutations in expression vectors by the gapped-duplex DNA method using alternating selectable markers. Nucleic Acids Res 17:4441–4454
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76:4350–4354
Vasantha N, Thompson LD (1986) Secretion of a heterologous protein fromBacillus subtilis with the aid of protease signal sequences. J Bacteriol 165:837–842
Vasantha N, Thompson LD, Rhodes C, Banner C, Nagle J, Filpula D (1984) Genes for alkaline protease and neutral protease fromBacillus amyloliquefaciens contain a large open reading frame between the regions coding for signal sequence and mature protein. J Bacteriol 159:811–819
Wells JA, Ferrari E, Henner DJ, Estell DA, Chen EY (1983) Cloning, sequencing, and secretion ofBacillus amyloliquefaciens subtilisin inBacillus subtilis. Nucleic Acids Res 11:7911–7925
Wells JA, Powers DB, Bott RR, Katz BA, Ultsch MH, Kossiakoff AA, Power SD, Adams RM, Heyneker HH, Cunningham BC, Miller JV, Graycar TP, Estell DA (1987a) Protein engineering of subtilisin. In: Oxender DL, Fox CF (eds) Protein engineering. Alan R Liss, New York, pp 279–287
Wells JA, Cunningham BC, Graycar TP, Estell DA (1987b) Recruitment of substrate-specificity properties from one enzyme into a related one by protein engineering. Proc Natl Acad Sci USA 84:5167–5171
Wong SL, Doi RH (1986) Determination of the signal peptidase cleavage site in the preprosubtilisin ofBacillus subtilis. J Biol Chem 261:10176–10181
Author information
Authors and Affiliations
Additional information
Communicated by J.W. Lengeler
Rights and permissions
About this article
Cite this article
Schülein, R., Kreft, J., Gonski, S. et al. Preprosubtilisin Carlsberg processing and secretion is blocked after deletion of amino acids 97-101 in the mature part of the enzyme. Molec. Gen. Genet. 227, 137–143 (1991). https://doi.org/10.1007/BF00260718
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00260718