Summary
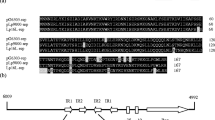
The sequence and genetic organization was determined of the 2508 by lactococcal portion of pFX2, which was derived from a crypticLactococcus lactis subsp.lactis plasmid and used as the basis for construction of a series of lactococcal vectors. A lactococcal plasmid plus origin and two replication protein-coding regions (repA andrepB) were located. RepA has a helix-turn-helix motif, a geometry typical of DNA-binding proteins. RepB shows a high degree of homology to the plasmid replication initiation proteins from other gram-positive bacteria andMycoplasma. The transcribed inverted repeat sequence betweenrepA andrepB could form an attenuator to regulate pFX2 replication. Upstream of theori site, and in a region which was non-essential for replication, a 215 by sequence identical to the staphylococcal plasmid pE194 and carrying the RSA site was identified. The genetic organization of this lactococcal plasmid replicon shares significant similarity with pE194 group plasmids.
Similar content being viewed by others
References
Alonso JC, Tailor RH (1987) Initiation of plasmid pC194 replication and its control inBacillus subtilis. Mol Gen Genet 210:476–484
Bates EEM, Gilbert HJ (1989) Characterization of a cryptic plasmid fromLactobacillus plantarum. Gene 85:253–258
Bergemann AD, Whitley JC, Finch LR (1989) Homology of mycoplasma plasmid pADB201 and staphylococcal plasmid pE194. J Bacteriol 171:593–595
Chou PY, Fasman GD (1974) Prediction of protein conformation. Biochemistry 13:222–245
Crow VL, Davey GP, Pearce LE, Thomas TD (1983) Plasmid linkage of the D-tagatose 6-phosphate pathway inStreptococcus lactis: effect in lactose and galactose metabolism. J Bacteriol 153:76–83
de la Campa AG, del Solar GH, Espinosa M (1990) Initiation of replication of plasmid pLS1. The initiator protein repB acts on two distant DNA regions. J Mol Biol 213:247–262
de Vos WM (1987) Gene cloning and expression in lactic streptococci. FEMS Microbiol Rev 46:281–295
del Solar GH, de la Campa AG, Perez-Martin J, Choli T, Espinosa M (1989) Purification and characterization of RepA, a protein involved in the copy number control of plasmid pLS1. Nucleic Acids Res 17:2405–2420
Doi RH (1984) Genetic engineering inBacillus subtilis. Biotechnol Genet Eng Rev 2:121–155
Garnier AL, Cole ST (1988) Complete nucleotide sequence and genetic organization of the bacteriogenic plasmid, pIP404, fromClostridium perfringens. Plasmid 19:134–150
Garvie EI, Farrow JAE, Phillips BA (1981) A taxonomic study of some strains of streptococci which grow at 10°C but not at 45°C includingStreptococcus lactis andStreptococcus cremoris. Zentralbl Bakteriol Hyg [C] 2:151–165
Gennaro ML, Kornblum J, Novick RP (1987) A site-specific recombination function inStaphylococcus aureus plasmids. J Bacteriol 169:2601–2610
Gruss A, Ehrlich SD (1989) The family of highly interrelated singlestranded deoxyribonucleic acid plasmids. Microbiol Rev 53:231–241
Harley CB, Reynolds RP (1987) Analysis ofE. coli promoter sequences. Nucleic Acids Res 15:2343–2361
Hayes F, Daly C, Fitzgerald GF (1990) Identification of the minimal replicon ofLactococcus lactis subsp.lactis UC317 plasmid pCI305. Appl Environ Microbiol 56:202–209
Horinouchi S, Weisblum B (1982) Nucleotide sequence and functional map of pE194, a plasmid that specifies inducible resistance to macrolide, lincosamide, and streptogramin type B antibiotics. J Bacteriol 150:804–814
Jordan SR, Pabo CO (1988) Structure of the lambda complex resolution: details of the repressor-operator interactions. Science 242:893–899
Kendall KJ, Cohen SN (1988) Complete nucleotide sequence of theStreptomyces lividans plasmid pIJ101 and correlation of the sequence with genetic properties. J Bacteriol 170:4634–4651
Kok J, van der Vossen JMBM, Venema G (1984) Construction of plasmid cloning vectors for lactic streptococci which also replicate inBacillus subtilis andStreptococcus lactis. Appl Environ Microbiol 48:726–731
Lakshmidevi G, Davidson BE, Hillier AJ (1990) Molecular characterization of promoters of theLactococcus lactis subsp.cremoris temperate bacteriophage BK5-T and identification of a phage gene implicated in the regulation of promoter activity. Appl Environ Microbiol 56:934–942
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY
Novick NP (1989) Staphylococcal plasmids and their replication. Annu Rev Microbiol 43:537–565
Novick RP, Iordanescu S, Projan SJ, Kornblum J, Edelman I (1989) pTl81 plasmid replication is regulated by a counterscript driven transcriptional attenuator. Cell 59:395–404
Ohlendorf DH, Anderson WF, Matthews BW (1983) Many gene-regulatory proteins appear to have a similar α-helical fold that binds DNA and evolve from a common precursor. J Mol Evol 19:109–114
Pabo CO, Sauer RT (1984) Protein-DNA recognition. Annu Rev Biochem 53:293–321
Praszkier J, Bird P, Nikoletti S, Pittard J (1989) Role of counter-transcript RNA in the copy number control system of an IncB miniplasmid. J Bacteriol 171:5056–5064
Priebe SD, Lacks SA (1989) Regions of the streptococcal plasmid pMV158 required for conjugative mobilization. J Bacteriol 171:4778–4784
Puyet A, del Solar GH, Espinosa M (1988) Identification of the origin and direction of the broad-host-range plasmid pLS1. Nucleic Acids Res 16:115–133
Rosenberg M, Court D (1979) Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet 13:319–353
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74:5463–5467
Scheer-Abramowitz J, Gryczan TJ, Dubnau D (1981) Origin and mode of replication of plasmid pE194 and pUB110. Plasmid 6:67–77
Tomizawa J (1986) Control of ColEl plasmid replication: initial interaction of RNA I and the primer transcript is reversible. Cell 40:527–535
van der Lelie D, Bron S, Venema G, Oskam L (1989) Similarity of minus origins of replication and flanking open reading frames of plasmids pUB110, pTB913 and pMV158. Nucleic Acids Res 17:7283–7294
van der Vossen JMBM, van der Lelie D, Venema G (1987) Isolation and characterization ofStreptococcus cremoris Wg2-specific promoters. Appl Environ Microbiol 53:2452–2457
Villafane R, Bechofer CH, Narayanan CS, Dubnau D (1987) Replication control genes of plasmid pE194. J Bacteriol 169:4822–4829
von Wright A, Wessels A, Tynkkynen S, Saarela M (1990) Isolation of a replication region of a large lactococcal plasmid and use in cloning of a nisin resistance determinant. Appl Environ Microbiol 56:2029–2035
Willetts N, Wilkins B (1984) Processing of plasmid DNA during bacterial conjugation. Microbiol Rev 48:24–41
Xu F, Pearce LE, Yu PL (1990) Molecular cloning of a proteinase gene fromLactococcus lactis subsp.cremoris and construction of a new lactococcal vector pFX1. Arch Microbiol 154:99–104
Xu F, Pearce LE, Yu PL (1991) Construction of a family of lactococcal vectors for gene cloning and translational fusion. FEMS Microbiol Lett 77:55–60
Yanisch-Perron C, Vieira J, Messing J (1983) Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mpl8 and pUC19 vectors. Gene 33:103–119
Author information
Authors and Affiliations
Additional information
Communicated by W. Goebel
Rights and permissions
About this article
Cite this article
Xu, F., Pearce, L.E. & Yu, PL. Genetic analysis of a lactococcal plasmid replicon. Molec. Gen. Genet. 227, 33–39 (1991). https://doi.org/10.1007/BF00260703
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00260703