Summary
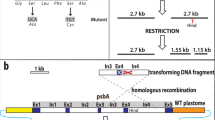
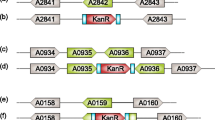
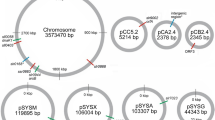
In a temperature-sensitive, high CO2-requiring mutant of Synechococcus sp. PCC7942, the ability to fix intracellularly accumulated inorganic carbon was severely impaired at non-permissive temperature (41° C). In contrast, inorganic carbon uptake and ribulose-1,5-bisphosphate carboxylase activity in the mutant were comparable to the respective values obtained with the wild-type strain. The mutant was transformed to the wild-type phenotype (ability to form colonies at non-permissive temperature under ordinary air) with the genomic DNA of the wild-type strain. A clone containing a 36 kb genomic DNA fragment of the wild-type strain complemented the mutant phenotype. The complementing activity region was associated with internal 17 kb SmaI, 15 kb HindIII, 3.8 kb BamHI and 0.87 kb Pstl fragments. These 4 fragments overlapped only in a 0.4 kb HindIII-PstI region. In the transformants obtained with total genomic DNA or a plasmid containing the 3.8 kb BamHI fragment, the ability to fix intracellular inorganic carbon was restored. Southern hybridization and partial nucleotide sequence analysis indicated that the cloned genomic region was located approximately 20 kb downstream from the structural genes for subunits of ribulose-1,5-bisphosphate carboxylase/oxygenase. The cloned region was transcribed into a 0.5 kb mRNA. These results indicate that the cloned genomic region of Synechococcus sp. PCC7942 is involved in the efficient utilization of intracellular inorganic carbon for photosynthesis.
Similar content being viewed by others
References
Abe T, Tsuzuki M, Miyachi S (1987) Transport and fixation of inorganic carbon during photosynthesis in cells of Anabaena grown under ordinary air. III. Some characteristics of the HCO3 −-transport system in cells grown under ordinary air. Plant Cell Physiol 28:867–874
Abe T, Tsuzuki M, Kadokami Y, Miyachi S (1988) Isolation and characterization of temperature-sensitive, high-CO2 requiring mutant of Anacystis nidulans R2. Plant Cell Physiol 29:1353–1360
Aiba H, Adhya S, De Crombrugghe B (1981) Evidence for two functional gal promoters in intact Escherichia coli cells. J Biol Chem 256:11905–11910
Aizawa K, Miyachi S (1986) Carbonic anhydrase and CO2 concentrating mechanisms in microalgae and cyanobacteria. FEMS Microbiol Rev 39:215–233
Badger MR, Andrews TJ (1982) Photosynthesis and inorganic carbon usage by the marine cyanobacterium, Synechococcus sp. Plant Physiol 70:517–523
Badger MR, Kaplan A, Berry JA (1980) Internal inorganic carbon pool of Chlamydomonas reinhardtii. Evidence for a carbon dioxide-concentrating mechanism. Plant Physiol 66:407–413
Cooper TG, Filmer D (1969) The active species of “CO2” utilized by ribulose diphosphate carboxylase. J Biol Chem 244:1081–1083
Davis GD, Dibner MD, Battey JF (1986) Basic methods in molecular biology. Elsevier, New York
Friedberg D, Kaplan A, Ariel R, Kessel M, Sejffers J (1989) The 5′-flanking region of the gene encoding the large subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase is crucial for growth of the cyanobacterium Synechococcus sp. strain PCC7942 at the level of CO2 in air. J Bacteriol 171:6069–6076
Golden SS, Brusslan J, Haselkorn R (1987) Genetic engineering of the cyanoacterial chromosome. Methods Enzymol 153:215–231
Hanahan D (1985) Techniques for transformation of E. coli. In: Glover DM (ed) DNA cloning: a practical approach, vol 1. IRL Press, Oxford, pp 109–135
Kaplan A, Badger MR, Berry JA (1980) Photosynthesis and the intracellular inorganic carbon pool in the blue-green alga Anabaena variabilis: response to external CO2 concentration. Planta 149:219–226
Kuhlemeier CJ, Van Arkel GA (1987) Host-vector systems for gene cloning in cyanobacteria. Methods Enzymol 153:199–215
Lau RH, Straus NA (1985) Versatile shuttle cloning vectors for the unicellular cyanobacterium Anacystis nidulans R2. FEMS Microbiol Lett 27:253–256
Mackinney G (1941) Absorption of light by chlorophyll solutions. J Biol Chem 140:315–322
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning, a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York
Marcus Y, Schwarz R, Friedberg D, Kaplan A (1986) High CO2 requiring mutant of Anacystis nidulans R2. Plant Physiol 82:610–612
Miller AG, Colman B (1980) Active transport and accumulation of bicarbonate by a unicellular cyanobacterium. J Bacteriol 143:1253–1259
Ogawa T, Kaneda T, Omata T (1987) A mutant of Synechococcus PCC7942 incapable of adapting to low CO2 concentration. Plant Physiol 84:711–715
Ogawa T, Williams JGK, Omata T (1990) Molecular analysis of mutants of Synechocystis PCC6803 defective in inorganic carbon transport. In: Baltscheffsky M (ed) Current research in photosynthesis, vol IV. Kluwer Academic Publishers, Dordrecht, pp 471–474
Pierce J, Carlson TJ, Williams JGK (1989) A cyanobacterial mutant requiring the expression of ribulose bisphosphate carboxylase from a photosynthetic anaerobe. Proc Natl Acad Sci USA 86:5753–5757
Price GD, Badger MR (1989a) Ethoxyzolamide inhibition of CO2 uptake in the cyanobacterium Synechococcus PCC7942 without apparent inhibition of internal carbonic anhydrase activity. Plant Physiol 89:37–43
Price GD, Badger MR (1989b) Isolation and characterization of high CO2-requiring mutants of the cyanobacterium Synechococcus PCC7942. Plant Physiol 91:514–525
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74:5463–5467
Schwarz R, Friedberg D, Kaplan A (1988) Is there a role for the 42 kilodalton polypeptide in inorganic carbon uptake by cyanobacteria? Plant Physiol 88:284–288
Shinozaki K, Sugiura M (1983) The gene for the small subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase is located close to the gene for the large subunit in the cyanobacterium Anacystis nidulans 6301. Nucleic Acids Res 11:6957–5964
Shinozaki K, Yamada C, Takahata N, Sugiura M (1983) Molecular cloning and sequence analysis of the cyanobacterial gene for the large subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase. Proc Natl Acad Sci USA 80:4050–4054
Shiraiwa Y, Miyachi S (1985) Role of carbonic anhydrase in photosynthesis of blue-green alga (cyanobacterium) Anabaena variabilis ATCC29413. Plant Cell Physiol 26:109–116
Suzuki E, Tsuzuki M, Miyachi S (1987) Photosynthetic characteristics of chloroplasts isolated from Euglena gracillis Z grown photoautotrophically. Plant Cell Physiol 28:1377–1388
Suzuki E, Fukuzawa H, Abe T, Miyachi S (1990) Identification of the genomic region which complements a temperature-sensitive, high-CO2 requiring mutant of the cyanobacterium, Synechococcus PCC7942. In: Baltscheffsky M (ed) Current research in photosynthesis, vol IV. Kluwer Academic Publishers, Dordrecht, pp 467–470
Volokita M, Zenvirth D, Kaplan A, Reinhold L (1984) Nature of the inorganic carbon species actively taken up by the cyanobacterium Anabaena variabilis. Plant Physiol 76:599–602
Williams JGK, Szalay AA (1983) Stable integration of foreign DNA into the chromosome of the cyanobacterium Synechococcus R2. Gene 24:37–51
Yanisch-Perron C, Vieira J, Messing J (1985) Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103–119
Author information
Authors and Affiliations
Additional information
Communicated by R.G. Herrmann
Rights and permissions
About this article
Cite this article
Suzuki, E., Fukuzawa, H. & Miyachi, S. Identification of a genomic region that complements a temperature-sensitive, high CO2-requiring mutant of the cyanobacterium, Synechococcus sp. PCC7942. Molec. Gen. Genet. 226, 401–408 (1991). https://doi.org/10.1007/BF00260652
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00260652