Summary
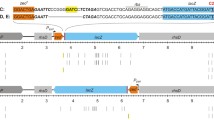
Ochre suppressor mutations induced by UV in the Escherichia coli glnU tRNA gene are CG to TA transitions at the first letter of the anticodon-encoding triplet, CAA. Premutational UV photoproducts at this site have long been known to exhibit an excision repair anomaly (“mutation frequency decline” or MFD), whereby post-irradiation inhibition of protein synthesis enhances their excision and reduces suppressor mutation yields ten-fold. We sought to clarify the basis of this unique repair response by determining the spectrum of UV photoproducts on both strands of a 36 by region of glnU which includes the anticodon-encoding triplet. We found that four different photolesions are produced within the 3 by sequence corresponding to the tRNA anticodon: (i) on the transcribed strand, TC (6–4) photoproducts and TC cyclobutane dimers are formed in equal numbers at the site of the C to T transition, indicating that this site is a hotspot for the usually less frequent (6–4) photoproduct; (ii) on the nontranscribed strand, TT dimers are found opposite the second and third letters of the anticodon-encoding triplet, adjacent to the mutation site; and (iii) on the nontranscribed strand, an alkali-sensitive lesion other than a (6–4) photoproduct is formed, apparently at the G in the mutation site. We suggest that mutation frequency decline may reflect excision repair activity at closely spaced UV lesions on opposite strands, resulting in double-strand breaks and the death of potential mutants.
Similar content being viewed by others
References
Bockrath R, Cheung MK (1973) The role of nutrient broth supplementation in UV mutagenesis of E. coli. Mutat Res 19:23–26
Bockrath RC, Palmer JE (1977) Differential repair of premutational UV-lesions at tRNA genes in E. coli. Mol Gen Genet 156:133–138
Bockrath R, Ruiz-Rubio M, Bridges BA (1987) Specificity of mutation by UV light and delayed photoreversal in umuC-defective Escherichia coli K-12: a targeting intermediate at pyrimidine dimers. J Bacteriol 169:1410–1417
Bockrath R, Hodes MZ, Valerie K, Riel JK de (1988) UV mutagenesis in E. coli with excision repair initiated by uvrABC or denV gene products. Mutat Res 193:87–96
Brash DE (1988) Quantitating DNA lesions at the DNA sequence level. In: DNA repair: A laboratory manual of research procedures, vol 3. Marcel Dekker, New York
Brash DE, Haseltine WA (1982) UV-induced mutation hotspots occur at DNA-damage hotspots. Nature 298:189–192
Bridges BA, Dennis RE, Munson RJ (1967) Differential induction and repair of ultraviolet damage leading to true reversion and external suppressor mutations of an ochre codon in Escherichia coli B/r WP2. Genetics 57:897–908
Doudney CO (1976) The two-lesion hypothesis for UV-induced mutation in relation to recovery of capacity for DNA replication. In: Hanawalt PC, Setlow RB (eds) Molecular mechanisms for repair of DNA, vol 1. Plenum, New York, p 389
Duker NJ, Gallagher PE (1988) Purine photoproducts. Photochem Photobiol 48:35–39
Engstrom JE, Bockrath RC (1980) Mutation frequency decline in a rel strain of Escherichia coli B/r. Mol Gen Genet 178:143
Engstrom J, Larsen S, Rogers S, Bockrath R (1984) UV-mutagenesis at a cloned target sequence: converted suppressor mutation is insensitive to mutation frequency decline regardless of the gene orientation. Mutat Res 132:143–151
George D, Witkin EM (1974) Slow excision repair in an mfd mutant of Escherichia coli B/r. Mol Gen Genet 133:23–30
George D, Witkin EM (1975) Ultraviolet light-induced responses of an mfd mutant of Escherichia coli B/r having a slow rate of dimer excision. Mutat Res 28:347–354
Glickman BW, Schaaper RM, Haseltine WA, Dunn RL, Brash DE (1986) The C-C (6–4) photoproduct is mutagenic in Escherichia coli. Proc Natl Acad Sci USA 83:6945–6949
Hanahan D (1983) Studies on transformation of Escherichia coli with plasmids. J Mol Biol 166:557–582
Lam LH, Reynolds RJ (1987) DNA sequence dependence of closely opposed cyclobutyl pyrimidine dimers induced by UV radiation. Mutat Res 178:167–175
Loeb LA, Kunkel TA (1982) Fidelity of DNA synthesis. Annu Rev Biochem 52:429–457
Mandecki W (1986) Oligonucleotide-directed double-strand break repair in plasmid of Escherichia coli: A method for site-specific mutagenesis. Proc Natl Acad Sci USA 83:7177–7181
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning — a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York
Messing J, Vieira J (1982) A new pair of M13 vectors for selecting either DNA strand of double digest restriction fragments. Gene 19:269–284
Miller JH (1985) Mutational specificity in bacteria. Annu Rev Genet 17:215–238
Osborn M, Person S (1967) Characteristics of revertants of E. coli WU36-10 and WP2 using amber mutants and an ochre mutant of bacteriophage T4. Mutat Res 4:504–512
Sancar A, Sancar GB (1988) DNA repair enzymes. Annu Rev Biochem 57:29–67
Schaaper R, Dunn RL, Glickman BW (1987) Mechanisms of ultraviolet-induced mutation: Mutational spectra in the Escherichia coli lacI gene for a wild type and excision-repair-deficient strain. J Mol Biol 198:187–202
Setlow JK, Boling ME (1970) Ultraviolet action spectra for mutation in Escherichia coli. Mutat Res 9:437–442
Snow ET, Foote RS, Mitra S (1984) Kinetics of incorporation of O 6-methyldeoxyguanosine monophosphate during in vitro DNA synthesis. Biochemistry 23:4289–4294
Walker GC (1984) Inducible DNA repair systems. Annu Rev Biochem 54:425–457
Witkin EM (1966a) Radiation-induced mutations and their repair. Science 152:1345–1352
Witkin EM (1966b) Mutation and the repair of radiation damage in bacteria. Radiat Res (Suppl) 6:30–53
Witkin EM (1969) Ultraviolet-induced mutation and DNA repair. Annu Rev Genet 3:525–551
Witkin EM (1975) Persistence and decay of thermoinducible error-prone repair activity in nonfilamentous derivatives of tif-1 Escherichia coli B/r: The timing of some critical events in ultraviolet mutagenesis. Mol Gen Genet 142:87–103
Witkin EM (1976) Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev 40:869–907
Author information
Authors and Affiliations
Additional information
Communicated by R. Devoret
Rights and permissions
About this article
Cite this article
Garvey, N., Witkin, E.M. & Brash, D.E. Ultraviolet photoproducts at the ochre suppressor mutation site in the gln U gene of Escherichia coli: Relevance to “mutation frequency decline”. Mol Gen Genet 219, 359–364 (1989). https://doi.org/10.1007/BF00259607
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00259607