Summary
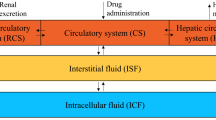
The kinetics of methotrexate were studied in a group of 59 patients after infusion of high doses. From the triexponential analysis of the serum concentration curve, rate constants and relative concentrations in non-circulating pools were calculated, using a linear ‘mammillary’ three-compartment model. No significant variation as a function of dose (300–6,000 mg/m2) was observed. In circumstances the rapidly exchanging pool was a reflection of the circulating pool, suggesting that it is an extravascular, protein-bound pool of methotrexate. Considerable variations of the relative concentration and rate constants of the slowly exchanging pool were observed to be a function of the duration of methotrexate infusion, the presence of other drugs, and the patient's age. If this compartment is assumed to include the target cells for methotrexate, then the large variations observed could be responsible for individual differences in toxicity and/or effectiveness.
Similar content being viewed by others
References
Bishoff KB, Dedrick RL, Zaharko DS, Longstreth JA (1971) Methotrexate pharmacokinetics. J Pharm Sci 60:1128
Buice RG, Evans NE, Karas J, Nicholas GA, Sidhu P, Straughn AB, Meyer MC, Crom WR (1980) Evaluation of enzyme immunoassay, radioassay and radioimmunoassay of serum methotrexate, as compared with chromatography. Clin Chem 26:1902
Cohen HJ, Jaffe N (1976) Pharmacokinetic and clinical studies of 24 h infusions of high-dose methotrexate. Cancer Chemother Pharmacol 1:61
Djerassi I (1967) Methotrexate infusions and intensive supportive care in the management of children with acute lymphocyte leukemia: follow-up report. Cancer Res 27:2561
Evans WE, Pratt CB, Taylor RH, Barker LF, Crom WR (1979) Pharmacokinetic monitoring of high-dose methotrexate. Cancer Chemother Pharmacol 3:161
Goh TS, Wong KY, Lampkin B, O'Leary J, Gnarra D (1979) Evaluation of 24 hour infusion of high-dose methotrexate: pharmacokinetics and toxicity. Cancer Chemother Pharmacol 3:177
Goldie TS, Price LA, Harrap KR (1978) Methotrexate toxicity: correlation with duration of administration, plasma levels, dose and excretion pattern. Eur J Cancer 8:409
Huffmann DH, Wan SH, Azarnoff DL, Hoogstraten B (1973) Pharmacokinetics of high-dose methotrexate. Clin Pharmacol Ther 14:572
Isacoff WH, Morrison PF, Aroesty J, Willis KL, Block JB, Lincoln TL (1977) Pharmacokinetics of high dose methotrexate-citrovorum factor rescue. Cancer Treat Rep 61:1665
Jaffe N, Howell S (1979) Antifolate rescue. Use of high-dose methotrexate and citrovorum factor. In: Advances in cancer chemotherapy. Marcel Dekker, New York Basel, p 111
Jaffe N, Frei E III, Traggis D, Watts H (1977) Weekly high-dose methotrexate-citrovorum factor in osteogenic sarcoma. Cancer 39:45
Leme PR, Creaven PJ, Allen LM, Berman M (1975) Kinetic model for the disposition and metabolism of moderate and high-dose methotrexate (NSC-740) in man. Cancer Chemother Rep 59:811
Monjanel S, Rigault JP, Cano JP, Carcassonne Y, Faure R (1979) High-dose methotrexate: preliminary evaluation of a pharmacokinetic approach. Cancer Chemother Pharmacol 3:189
Pitman SW, Parker LM, Tatterstall MHM, Jaffe N, Frei E III (1975) Clinical trials of high-dose methotrexate (NSC-740) with citrovorum factor (NSC-3590)-Toxicologic and therapeutic observations. Cancer Chemother Rep 6:43
Reich SD (1979) Mathematical modeling-Guide to high-dose methotrexate infusion therapy. Cancer Chemother Pharmacol. 3:25
Rosen G, Tan C, Sammaneechi A, Beattie EL Jr, Marcove RC, Murphy ML (1975) The rationale for multiple drug chemotherapy in the treatment of osteogenic sarcoma. Cancer 35:936
Stoller RG, Jacob SA, Drake JC, Lutz RJ, Chabner BA (1975) Pharmacokinetics of high-dose methotrexate (NSC-740). Cancer Chemother Rep 6:19
Wang JJ, Freeman AI, Sinks LF (1976) Treatment of acute lymphocytic leukemia by high-dose intravenous methotrexate. Cancer Res 36:1441
Wang YM, Sutow WW, Romsdahl MM, Perez C (1979) Age-related pharmacokinetics of high-dose methotrexate in patients with osteosarcoma. Cancer Treat Rep 63:405
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lokiec, F., Poirier, O., Gisselbrecht, C. et al. Effect of combination chemotherapy, duration of methotrexate administration, and Patient's age on methotrexate pharmacokinetics. Cancer Chemother. Pharmacol. 9, 165–168 (1982). https://doi.org/10.1007/BF00257746
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00257746