Abstract
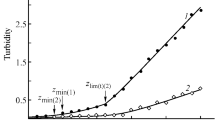
Chitosan and polyacrylic acid mixtures were prepared in different mole ratios and at different pH values and ionic strengths (0.025–0.300). Complex formation was detected by turbidity measurement and quantified by weighing the freeze dried pellet recovered by centrifugation. No insoluble complex formation at pH=2 was detected. In the 3 to 6 pH range, the maximum complex formation occurred at different mole ratios. Quantitative analysis of the supernatant showed that pH affects the complex composition. Solution ionic strength in the 0.025–0.300 range did not affect complex formation.
Supernatant pH measurement showed that in the 3 to 5 pH range, the pH of the mixture decreased as the complex was formed. At pH=6, the opposite behavior was observed. This information was used to propose a mechanism for complex formation which was confirmed by quantitative analysis of the supernatant and IR analysis of the insoluble complex. These studies showed that an electrostatic interaction between COO− and NH3 + groups was involved in complex formation.
Similar content being viewed by others
References
H. Fukuda, “Polyelectrolyte complexes of sodium carboxymethylcellulose with chitosan”, Makromol. Chem. 180: 1631–1633 (1979).
Y. Kikuchi and A. Noda, “Polyelectrolytic complexes of heparin with chitosan”, J. of Appl. Polym. Sci. 20: 2561–2563, (1976).
S. Hirano, C. Mizutani, R. Yamaguchi, and O. Miura, “Formation of the polyelectrolyte complexes of some acidic glycosaminoglycans with partially N-acylated chitosans”, Biopolymers 17: 805–810, (1978).
P.V. Prabhu, A.C. Radhakrishnan, T.S.G. Iyer, “Chitosan as a water clarifying agent”, Fish Technol. 13 (1): 69–72, (1976).
A. Milazzo, “Use of chitosan as a flocculant in industrial effluent from lobster-processing plants”, Riv. Mercel. 21, (4): 349–54, (1982).
W.A. Bough, D.R. Lanoles, J. Miller, C.T. Young and T.R. McWhorten, “Utilization of chitosan for recovery of coagulated by-products from food processing wastes and treatment systems”, EPA-600-76-224, Sixth Proc. National Symp. Food Process. Wastes, p. 22–48, (1976).
N. Sutterlin, “Concentration dependence of the viscosity of dilute polymer solutions Huggins and Schulz-Blasehke coefficients”, In Polymer Handbook, 2nd ed., Brandrup J. and Immergut, E.H. (eds.). John Wiley & Sons, New York, NY, (1975).
C.A. Lang, “Simple microdetermination of Kjeldahl nitrogen in biological materials”, Anal. Chem. 30: 1692, (1958).
M. Nagasawa, T. Murase, K. Kondo, “Potentiometric titration of stereoregular polyelectrolytes”, J. Phys. Chem. 69:4005, (1965)
C.A. Kienzle-Sterzer, “Hydrodynamic behavior of a cationic polyelectrolyte”, Ph.D. thesis, Massachusetts Inst. of Technology, Cambridge, (1984).
A.B. Zezin, V.B. Rogacheva, V.S. Komanov and Razvodovskii, “The formation of amide linkages in polyelectrolyte salt complexes”, Vysokomol. Soyed. A17, (12): 2637–2643, (1975)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chavasit, V., Kienzle-Sterzer, C. & Antonio Torres, J. Formation and characterization of an insoluble polyelectrolyte complex: Chitosan-polyacrylic acid. Polymer Bulletin 19, 223–230 (1988). https://doi.org/10.1007/BF00255376
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00255376