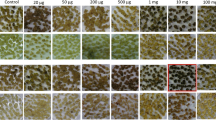
Summary
S-(2-aminoethyl-)L-cysteine and L-canavanine were less toxic for octopine-type crown gall tissues that contained lysopine dehydrogenase than for other crown gall or habituated tissues. These analogs are substrates for lysopine dehydrogenase in vitro and in vivo. Thus toxic analogs of amino acid precursors of opines may be useful in selecting for cells that contain an opine dehydrogenase.
Similar content being viewed by others
References
Birnberg P, Lippincott B, Lippincott J (1977) Two octopine dehydrogenases in crown-gall tumor tissue. Phytochemistry 16:647–650
Braun AC, Wood HN (1976) Suppression of the neoplastic state with the acquisition of specialized functions in cells, tissues, and organs of crown gall teratomas of tobacco. Proc Natl Acad Sci USA 73:496–500
Bright SWJ, Featherstone LC, Miflin BJ (1979) Lysine metabolism in a barley mutant resistant to S-(2-aminoethyl-)cysteine. Planta 146:629–633
Cattoir A, DeGryse E, Jacobs M, Negrutiu I (1980) Inhibition of barley and Arabidopsis callus growth by lysine analogues. Plant Sci Lett 17:327–332
Chaleff RS, Carlson PS (1975) Higher plant cells as experimental organisms. In: Ledoux L (eed) Genetic manipulations with plant material. Plenum Press, New York, pp 351–363
Easley CW (1965) Combinations of specific color reactions useful in the peptide mapping technique. Biochim Biophys Acta 107:386–388
Firmin JL, Fenwick GR (1978) Agropine —a major new plasmid-determined metabolite in crown gall tumors. Nature 276:842–844
Gelvin SB, Thomashow MF, McPherson JC, Gordon MP, Nester EW (1982) Sizes and map positions of several plasmid-DNA-encoded transcripts in octopine-type crown gall tumors. Proc Natl Acad Sci USA 79:76–80
Goldmann A (1977) Octopine and nopaline dehydrogenases in crown gall tumours. Plant Sci Lett 10:49–58
Hack E, Kemp JD (1977) Comparison of octopine, histopine, lysopine and octopinic acid synthesizing activities in sunflower crown gall tissues. Biochem Biophys Res Commun 78:785–791
Jones RG, Kornfled EC, McLaughlin KC (1950) The synthesis of some analogs of histamine and histidine containing the thiazole nucleus. J Am Chem Soc 72:4526–4529
Kahl G, Schell JS (eds) (1982) Molecular biology of plant tumors. Academic Press, New York London Paris
Kemp JD (1982) Enzymes in octopine and nopaline metabolism. In: Kahl G, Schell JS (eds) Academic Press, New York London Paris, pp 461–474
Lejeune B (1967) Etude de la synthèse de lysopine in vitro par des extraits de crown-gall. C R Acad Sci, Ser D 265: 1753–1755
Linsmaier EM, Skoog F (1965) Organic growth factor requirements of tobacco tissue cultures. Physiol Plant 8:100–127
Meins F, Jr (1982) Habituation of cultured plant cells. In: Kahl G, Schell JS (eds) Molecular biology of plant tumors. Academic Press, New York London Paris, pp 3–31
Otten LABM (1979) Lysopine dehydrogenase and nopaline dehydrogenase from crown gall tumour tissues. PhD Thesis, University Leyden, The Netherlands
Otten LABM, Schilperoort RA (1978) A rapid micro scale method for the detection of lysopine and nopaline dehydrogenase. Biochim Biophys Acta 527:497–500
Petit A, Morel G (1966) Le métabolisme de l'homoarginine par les tissus de Crown-Gall. C R Soc Biol (Paris) 160:1806–1807
Petit A, Tempé J, Morel G (1968) Sur la présence d'un produit de transformation de la canavanine dans les tissus tumoraux de Canavalia ensiformis. C R Soc Biol (Paris) 162: 632–634
Rosenthal GA (1977) The biological effects and mode of action of L-canavanine, a structural analogue of L-arginine. Q Rev Biol 52:155–178
Sacristan MD; Melchers G (1977) Regeneration of plants from habituated and Agrobacterium-transformed single-cell clones of tobacco. Mol Gen Genet 152:111–117
Thomashow MF, Nutter RC, Montoya AL, Gordon MP, Nester EW (1980) Integration and organization of T-DNA in crown gall tumors. Cell 19:729–739
Van Slogteren GMS, Hooykaas PJJ, Planqué K, de Groot B (1982) The lysopinedehydrogenase gene used as a marker for selection of octopine crown gall cells. Plant Mol Biol 1:133–142
White DWR, Vasil IK (1979) Use of amino acid analogueresistant cell lines for selection of Nicotiana sylvestris somatic cell hybrids. Theor Appl Genet 55:107–112
Yamada S, Itano HA (1966) Phenanthrenequinone as an analytical reagent for arginine and other monosubstituted guanidines. Biochim Biophys Acta 130:538–540
Author information
Authors and Affiliations
Additional information
Communicated by P. Maliga
Rights and permissions
About this article
Cite this article
Dahl, G.A., Tempé, J. Studies on the use of toxic precursor analogs of opines to select transformed plant cells. Theoret. Appl. Genetics 66, 233–239 (1983). https://doi.org/10.1007/BF00251151
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00251151