Summary
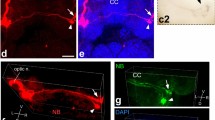
A comparison of the retinofugal projections in 14 species of plethodontid salamanders by means of the horseradish peroxidase (HRP) technique revealed almost identical contralateral projections. In all species studied three optic tracts were found. Behind the chiasma opticum the basal optic tract runs to the peduncle region, there forming the basal optic neuropil. The marginal optic tract courses from the chiasma over the thalamus to the tectum opticum where it covers the entire surface. In the anterior thalamus the marginal optic tract innervates the neuropil Bellonci-pars lateralis and the corpus geniculatum thalamicum, and more caudally the neuropil posterior thalami. The medial optic tract supplies the neuropil Bellonci-pars lateralis and pars medialis in the anterior thalamus from where it runs medial to the marginal optic tract as a separate tract to the uncinate field in the posterior thalamus.
The ipsilateral projections show differences among the species studied, although the global organization remains constant. The differences mainly concern the marginal optic tract which varies from being weakly labeled and restricted to the rostral part of the tectum opticum, to being heavily labeled and innervating the entire tectum to its caudal edge. Species with the heaviest ipsilateral projections all belong to the plethodontid tribe Bolitoglossini, all of which show direct development, a highly projectile tongue, rather frontally oriented eyes and excellent depth perception. In these species the thalamic ipsilateral projection areas are equal in size and shape to the contralateral one. The ipsilateral projections to the tectum show two distinct layers, a superficial and a deep one, which intermingle with the contralateral projections. The two other ipsilateral tracts do not differ significantly among the plethodontid species: the medial optic tract is always heavily and the basal optic tract always weakly labeled.
Similar content being viewed by others
References
Ariëns-Kappers CU, Huber GC, Crosby EC (1936) The comparative anatomy of the nervous system of vertebrates. Including man. Hafner, New York
Bass AH, Northcutt RG (1981) Retinal recipient nuclei in the painted turtle. Chrysemys picta: An autoradiographic and HRP study. J Comp Neurol 199:97–112
Caldwell JH, Berman N (1977) The central projections in the retina in Necturus maculosus. J Comp Neurol 171:455–464
Crapon de Caprona M-D, Fritzsch B (1983) The development of the retinopetal nucleus olfacto-retinalis of two chichlid fish as revealed by horseradish peroxidase. Dev Brain Res 11:281–301
Ekström P (1982) Retinofugal projections in the eel, Anguilla anguilla L. (Teleostei), visualized by the cobalt-filling technique. Cell Tissue Res 225:507–524
Fritzsch B (1980) Retinal projections in European Salamandridae. Cell Tissue Res 213:325–341
Fritzsch B, Himstedt W, Crapon de Caprona M-D (1985) Visual projections in larval Ichthyophis kothaoensis (Gymnophiona, Amphibia) (Submitted)
Frost DO, So K-F, Schneider GE (1979) Postnatal development of retinal projections in Syrian hamster: A study using autoradiographic and anterograde degeneration techniques. Neuroscience 4:16–49
Graybiel AM (1975) Anatomical organization of retinotectal afferents in the cat: An autoradiographic study. Brain Res 96:1–23
Gruberg ER (1972) Optic fiber projections of the tiger salamander Ambystoma tigrinum. J Hirnforsch 14:399–411
Guillery RW, Updyke BV (1976) Retinofugal pathways in normal and albino axolotls. Brain Res 109:235–244
Hayhow WR, Webb C, Jervie A (1960) The accessory optic system in the rat. J Comp Neurol 115:187–215
Herrick CJ (1933) The amphibian forebrain. VI. Necturus. J Comp Neurol 58:1–288
Herrick CJ (1948) The brain of the tiger salamander Ambystoma tigrinum. The University of Chicago Press, Chicago
Himstedt W, Manteuffel G (1985) Retinal projections in the caecilian Ichthyophis kothaoensis (Amphibia, Gymnophiona). Cell Tissue Res 239:689–692
Itaya SK, Van Hoesen GW (1983) Retinal axons to the medial terminal nucleus of the accessory optic system in old world monkeys. Brain Res 269:361–364
Jackway IS, Riss W (1972) Retinal projections in the tiger salamander Ambystoma tigrinum. Brain Behav Evol 5:401–442
Jen LS, So K-F, Yew DT, Lee M (1983) An autoradiographic study of the retinofugal projections in the shark, Hemiscyllium plagiosum. Brain Res 274:135–139
Kennedy MC, Rubinson K (1977) Retinal projections in larval, transforming and adult sea lamprey, Petromyzon marinus. J Comp Neurol 171:465–480
Land PW, Lund RD (1979) Development of rats uncrossed retinotectal pathway and its relation to plasticity studies. Science 205:698–700
Lázár G (1971) The projection of the retinal quadrants on the optic centers in the frog. Acta Morphol Hung 19:325–334
Lázár G (1978) Application of the cobalt-filling technique to show retinal projections in the frog. Neuroscience 3:725–737
Leghissa S (1962) L' evoluzione del tetto ottico nei bassi vertebrati. Arch Anat Embriol 67:343–413
Levine RL (1980) An autoradiographic study of retinal projections in Xenopus laevis with comparison to Rana. J Comp Neurol 189:1–29
Lipp HP, Schwegler H (1980) Increased labelling of the brain structures after injection of HRP dissolved in a nonionic detergent (Nonidet P 40). Neurosci Lett 5:196
Lombard RE, Wake DB (1977) Tongue evolution in the lungless salamanders, family Plethodontidae. II. Function and evolutionary diversity. J Morphol 153:39–80
Meyer DL, Ebbesson SOE (1981) Retinofugal and retinopetal connections in the upside-down catfish (Synodontis nigriventris). Cell Tissue Res 218:389–401
Montgomery N, Fite KV, Bengston L (1981) The accessory optic system in Rana pipiens: Neuroanatomical connections and intrinsic organization. J Comp Neurol 203:595–612
Northcutt RG (1980) Retinal projections in the Australian lungfish. Brain Res 185:85–90
Northcutt RG, Butler AB (1974) Evolution of reptilian visual systems: Retinal projections in a nocturnal lizard, Gecko gecko. J Comp Neurol 157:453–466
Northcutt RG, Butler AB (1976) Retinofugal pathways in the longnose gar Lepisosteus osseus. J Comp Neurol 166:1–16
Rettig G (1984) Neuroanatomische Untersuchungen der visuellen Projektionen bei Salamandern (Ordnung Caudata). Dissertation, Universität Bremen
Rettig G, Fritzsch B, Himstedt W (1981) Development of the retinofugal neuropil areas in the brain of the alpine newt, Triturus alpestris. Anat Embryol 162:163–171
Rodieck RW (1979) Visual pathways. Ann Rev Neurosci 2:193–225
Roth G, Wake DB (1985) Trends in the functional morphology and sensorimotor control of feeding behavior in salamanders: An example of the role of internal dynamics in evolution. Acta Biotheor 34:175–192
Roth G, Grunwald W, Linke R, Rettig G, Rottluff B (1983) Evolutionary patterns in the visual system of lungless salamanders (fam. Plethodontidae). Arch Biol Med Exp 16:329–341
Scalia F, Knapp H, Halpern M, Riss W (1968) New observations on the retinal projection in the frog. Brain Behav Evol 1:324–353
Somogyi P, Hodgson AJ, Smith AD (1979) An approach to tracing neuron network in the cerebral cortex and basal ganglia. Combination of Golgi staining, retrograde transport of horseradish peroxidase and anterograde degeneration of synaptic buttons in the same material. Neuroscience 4:1805–1852
Springer AD, Landreth GE (1977) Direct ipsilateral projections in goldfish (Carassius auratus). Brain Res 124:533–537
Steedman JG, Stirling RV, Gaze RM (1979) The central pathways of optic fibers in Xenopus tadpoles. J Embryol Exp Morphol 50:199–215
Stirling RV, Brändle K (1982) Expansion of the visual projection to the tectum opticum of axolotls during metamorphosis. Dev Brain Res 5:343–345
Ströer WFH (1940) Das optische System beim Wassermolch (Triturus taeniatus). Acta Neerl Morphol 3:178–195
Takasuji K, Ito H, Masai H (1983) Ipsilateral retinal projections in Japanese quail, Coturnix coturnix japonica. Brain Res Bull 10:53–56
Wake DB (1966) Comparative osteology and evolution of the lungless salamanders, family Plethodontidae. Mem So Calif Acad Sci 4:1–111
Wilczynski W, Zakon H (1982) Transcellular transfer of HRP in the amphibian visual system. Brain Res 239:29–40
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rettig, G., Roth, G. Retinofugal projections in salamanders of the family Plethodontidae. Cell Tissue Res. 243, 385–396 (1986). https://doi.org/10.1007/BF00251055
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00251055