Summary
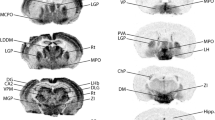
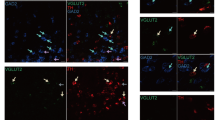
In situ hybridization histochemistry and RNA blots were used to study the expression of glutamic acid decarboxylase (GAD) mRNA in rats with or without a unilateral lesion of midbrain dopamine neurons. Two populations of GAD mRNA positive neurons were found in the intact caudate-putamen, substantia nigra and fronto-parietal cortex. In caudate-putamen, only one out of ten of the GAD mRNA positive neurons expressed high levels, while in substantia nigra every second of the positive neurons expressed high levels of GAD mRNA. Relatively few, but intensively labelled neurons were found in the intact fronto-parietal cerebral cortex. In addition, one out of six of the GAD mRNA positive neurons in the fronto-parietal cortex showed a low labeling. On the ipsilateral side, the forebrain dopamine deafferentation induced an increase in the number of neurons expressing high levels of GAD mRNA in caudateputamen, and a decrease in fronto-parietal cortex. A smaller decrease was also seen in substantia nigra. However, the total number of GAD mRNA positive neurons were not significantly changed in any of these brain regions. The changes in the levels of GAD mRNA after the dopamine lesion were confirmed by RNA blot analysis. Hence, midbrain dopamine neurons appear to control neuronal expression of GAD mRNA by a tonic down-regulation in a fraction of GAD mRNA positive neurons in caudate-putamen, and a tonic up-regulation in a fraction of GAD mRNA positive neurons in fronto-parietal cortex and substantia nigra.
Similar content being viewed by others
References
Balcom G, Lenox R, Meyerhoff J (1975) Regional γ-aminobuturic acid levels in rat brain determined after microwave fixation. J Neurochem 24:609–613
Berod A, Biguet NF, Dumas S, Bloch B, Mallet J (1987) Modulation of tyrosine hydroxylase gene expression in the central nervous system visualized by in situ hybridization. Proc Natl Acad Sci USA 84:1699–1703
Björklund A, Lindvall O (1984) Catecholaminergic brain stem regulatory systems. In: Björklund A, Hökfelt T (eds) Handbook of chemical neuroanatomy, Vol 2. Elsevier, New York, pp 55–122
Bolam JP, Ingham CA, Smith AD (1984) The section-Golgi-impregnation procedure. 3. Combination of Golgi-impregnation with immunohistochemistry and electron microscopy to characterize acetylcholinesterase-containing neurons in the rat neostriatum. Neuroscience 12:687–709
Brownstein MJ, Mroz EA, Tappaz ML, Leeman SE (1977) On the origin of substance P and glutamic acid decarboxylase (GAD) in the substantia nigra. Brain Res 135:315–323
Chesselet M-F (1984) Presynaptic regulation of neurotransmitter release in the brain: facts and hypothesis. Neuroscience 12:347–375
Chesselet M-F, Weiss L, Wuenschell C, Tobin AJ, Affolter H-U (1987) Comparative distribution of mRNAs for glutamic acid decarboxylase, tyrosine hydroxylase, and tachykinins in the basal ganglia: an in situ hybridization study in the rodent brain. J Comp Neurol 262:125–140
Christensen-Nylander I, Herrera-Marschitz M, Staines W, Hökfelt L, Terenius L, Ungerstedt U, Cuello C, Oertel WH, Goldstein M (1986) Striato-nigral dynorphin and substance P pathways in the rat. I. Biochemical and immunohistochemical studies. Exp Brain Res 54:169–192
Ernfors P, Hallböök F, Ebendahl T, Shooter EM, Radeke MJ, Misko TP, Persson H (1988) Developmental and regional expression of β-nerve growth factor receptor messenger RNA in the chick and rat. Neuron 1:983–996
Gale K, Casu M (1981) Dynamic utilization of GABA in substantia nigra: regulation by dopamine and GABA in the striatum, and its clinical and behavioural implications. Molec Cell Biochem 39:369–405
Garbutt JC, van Kammen DP (1983) The interaction between GABA and dopamine: implications for schizophrenia. Schizophr Bull 9:336–353
Girault JA, Spampinato U, Glowinski J, Besson MJ (1987) In vivo release of (3H)γ-aminobutyric acid in the rat neostriatum. II. Opposing effects of D1 and D2 dopamine receptor stimulation in the dorsal caudate putamen. Neuroscience 4:1109–1117
Haverstick DM, Rubenstein A, Bannon MJ (1989) Striatal tachykinin gene expression regulated by interaction of D-1 and D-2 dopamine receptors. J Pharmacol Exp Ther 248:858–862
Herrera-Marschitz M, Ungerstedt U (1984) Evidence that striatal efferents relate to different dopamine receptors. Brain Res 323:269–278
Hornykiewicz O (1982) Brain neurotransmitter changes in Parkinson's disease. In: Marsden CD, Fahn S (eds) Movement disorders. Butterworth Scientific, London, pp 41–58
Innis RB, Andrade R, Aghajanian GK (1985) Substance K excites dopaminergic and non-dopaminergic neurons in rat substantia nigra. Brain Res 335:381–383
Javoy-Agid F, Ploska A, Agid Y (1981) Microtopography of tyrosine hydroxylase, glutamic acid decarboylase, and choline acetyltransferase in the substantia nigra and ventral tegmental area of control and parkinsonian brains. J Neurochem 37:1218–1227
Kaufman DL, McGinnis JF, Krieger NR, Tobin AJ (1986) Brain glutamate decarboxylase cloned in λgt-11: fusion protein produces γ-aminobutyric acid. Science 232:1138–1140
Kim JS, Bak IJ, Hassler R, Okada Y (1971) Role of γ-aminobuturic acid (GABA) in the extrapyramidal system. Exp Brain Res 14:95–104
Kim YS, Thomas JW, Tillakaratne NJK, Monpied P, Suzdac PD, Banner C, Ginns E, Tobin AJ, Paul SM (1987) Glutamic acid decarboxylase mRNA in rat brain: regional distribution and effects of intrastriatal kaininc acid. Molec Brain Res 3:77–82
Kobayashi Y, Kaufman DL, Tobin AJ (1987) Glutamic acid decarboxylase cDNA: nucleotide sequence encoding an enzymatically active fusion protein J Neurosci 7:2768–2772
Krause JE, Chirgwin JM, Carter MS, Xu ZS, Hershey AD (1987) Three rat preprotachykinin mRNAs encode the neuropeptides substance P and neurokinin A. Proc Acad Sci USA 84:881–885
Kubota Y, Inagaki S, Kito S, Wu J-Y (1987) Dopaminergic axons make directly synapses with GABAregic neurons in the rat neostriatum. Brain Res 406:147–156
Lamouroux A, Faucon-Biquet N, Samolyk A. Privat A, Salomon JC, Pujol JF, Mallet J (1982) Identification of cDNA clones coding for rat tyrosine hydroxylase antigen. Proc Natl Acad Sci USA 79:3881–3885
Lindefors N, Brodin E, Tossman U, Segovia J, Ungerstedt U (1989) Tissue levels and in vivo release of tachykinins and GABA in striatum and substantia nigra of rat brain after unilateral striatal dopamine denervation. Exp Brain Res 74:527–534
McGeer PL, McGeer EG (1975) Evidence for glutamic acid decarboxylase-containing interneurons in the neostriatum. Brain Res 91:331–335
Mimy AJ, Caravatti M, Benoit R, Cohen A, Daubas P, Weydert A, Gros F, Buckingham ME (1981) Mouse actin messenger RNAs. J Biol Chem 256:1008–1041
Monfort JC, Javoy-Agid F, Hauw JJ, Dubois B, Agid Y (1985) Brain glutamate decarboxylase in Parkinson's disease with particular reference to premortem severity index. Brain 108:301–313
Morris BJ, Höllt V, Herz A (1988) Dopaminergic regulation of striatal proenkephaline mRNA and prodynorphin mRNA: contrasting effects of D1 and D2 antagonists. Neuroscience 25:525–532
Mugnaini E, Oertel WH (1985) An atlas of the distribution of GABAergic neurons and terminals in the rat CNS as revealed by GAD immunohistochemistry. In: Björklund A, Hökfelt T (eds) Handbook of neuroanatomy, Vol 4, Part I. Elsevier, Amsterdam, pp 436–608
Nagy JI, Vincent SR, Fibiger HC (1978) Altered neurotransmitter synthetic enzyme activity in some extrapyramidal nuclei after lesions of the nigro-striatal dopamine projection. Life Sci 22:1777–1782
Ottersen OP, Storm-Mathisen J (1984) Glutamate- and GABA-containing neurons in the mouse and rat brain, as demonstrated with a new immunocytochemical technique. J Comp Neurol 229:374–392
Paxinos S, Watson C (1982) The rat brain in stereotaxic coordinates. Academic Press, Australia
Perry TL, Javoy-Agid F, Agid Y, Fibiger H (1983) Striatal GABAergic activity is not reduced in Parkinson's disease. J Neurochem 40:1120–1123
Reiffenstein RJ, Neal MJ (1974) Uptake, storage, and release of γ-aminobuturic acid in normal and chronically denervated cat cerebral cortex. Can J Physiol Pharmacol 52:286–290
Pinnock RD, Dray A (1982) Differential sensitivity of presumed dopaminergic and non-dopaminergic neurons in rat substantia nigra to electrophoretically applied substance P. Neurosci Lett 29:153–158
Ribac CE (1978) Aspinous and sparsely-spinous stellate neurons in the visual cortex of rats contain glutamic acid decarboxylase. J Neurocytol 7:461–478
Schalling M, Hökfelt T, Wallace B, Goldstein M, Filier D, Yamin C, Schlesinger DH (1986) Tyrosine 3-hydroxylase in rat brain and adrenal medulla: hybridization histochemistry and immunohistochemistry combined with retrograde tracing. Proc Natl Acad Sci USA 83:6208–6212
Simpson GM, Cross AJ, Slater P, Deakin JFW (1988) Loss of cortical GABA uptake sites in Altzheimer's disease. J Neural Transm 71:219–226
Starr MS, Summerhayes M, Kilpatric IC (1983) Interactions between dopamine and γ-aminobuturate in the substantia nigra: implications for the striatonigral output hypothesis. Neuroscience 8:547–559
Tanaka Y, Niijima K, Mizuno Y, Yoshida M (1986) Changes in γ-buturate, glutamate, aspartate, glycine, and taurine contents in the striatum after unilateral nigrostriatal lesions in rat brain. Exp Neurol 91:259–268
Tappaz ML, Brownstein MJ, Palkowits M (1976) Distribution of glutamate decarboxylase in discrete brain nuclei. Brain Res 108:371–379
Ungerstedt U, Arbuthnott GW (1970) Quantitative recording of rotational behaviour in rats after 6-hydroxydopamine lesions of the nigrostriatal dopamine system. Brain Res 24:485–493
Ungerstedt U (1971) Stereotaxic mapping of the monoaminergic pathways in the rat brain. Acta Physiol Scand Suppl 367:1–48
Vernier P, Julien J-F, Rataboul P, Fourrier O, Feuerstein C, Mallet J (1988) Similar time course changes in striatal levels of glutamic acid decarboxylase and proenkephalin mRNA following dopaminergic deafferentation in the rat. J Neurochem 51:1375–1380
Waszczak BL, Walters JR (1984) A physiological role for dopamine as modulator of GABA effects in substantia nigra: supersensitivity in 6-hydroxydopamine-lesioned rats. Eur J Pharmacol 105:369–373
Wood TL, Franz GD, Menkes JH, Tobin AJ (1986) Regional distribution of messenger RNAs in postmortem human brain. J Neurosci Res 16:311–324
Young III WS, Bonner TI, Brann MR (1986) Mesencephalic dopamine neurons regulate the expression of neuropeptide mRNAs in the rat forebrain. Proc Natl Acad Sci USA 83:9827–9831
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lindefors, N., Brene, S., Herrera-Marschitz, M. et al. Region specific regulation of glutamic acid decarboxylase mRNA expression by dopamine neurons in rat brain. Exp Brain Res 77, 611–620 (1989). https://doi.org/10.1007/BF00249614
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00249614