Abstract
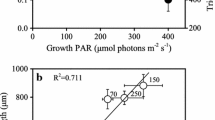
Examinations of the macromolecular components of the protein synthesizing system (RNA, DNA and protein) have been made in the marine cyanobacterium, Synechococcus sp. WH 7803. Slowly growing, irradiance limited cells have less RNA and lower rates of RNA synthesis than do those growing at rapid rates. RNA content and synthesis increase in conjunction with division rate. Protein content is variable. Protein synthesis increases up to a plateau at division rates less the maximum observed. The results imply that there is extra protein synthetic capacity produced at high, irradiance limited growth rates. Synechococcus sp. WH 7803 responds to an increase in irradiance through a rapid shiftup in macromolecular synthesis. RNA, protein and DNA increase in a sequential fashion which precedes the onset of cell division. After decreases in irradiance, protein synthesis is maintained despite reductions in RNA. This suggests that there is some degree of physiological buffering which occurs in this species. These studies indicate that, as in more extensively studied procaryotic models, the protein synthesizing system plays a central role in the global mechanisms regulating growth in Synechococcus sp. WH 7803.
Similar content being viewed by others
Abbreviations
- PSS:
-
protein synthesizing system
- HMW:
-
high molecular weight
- LMW:
-
low molecular weight
- TCA:
-
trichloroacetic acid
References
Adams DG, Phillips JM, Nichols JM, Carr NG (1977) The presence and absence of magic spot nucleotide modulation in cyanobacteria undergoing nutritional shift-down. FEBS Lett 81:48–52
Asato Y (1984) Characterization of cell cycle events in synchronized cultures of Anacystis nidulans. J Gen Microbiol 130:2535–2542
Armbrust EV, Bowen JD, Olson RJ, Chisolm SW (1989) Effect of light on the cell cycle of a marine Synechococcus strain. Appl Environ Microbiol 55:425–432
Barlow RG, Alberte RS (1985a) Photosynthetic characteristics of phycoerythrin-containing marine Synechococcus spp. I. Responses to growth photon flux density. Mar Biol 86:63–74
Barlow RG, Alberte RS (1985b) Photosynthetic characteristics of phycoerythrin-containing marine Synechococcus spp. II. Time course responses of photosynthesis to photo-inhibition. Mar Ecol Prog Ser 39:191–196
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 2:248–54
Chisolm SW, Armbrust EV, Olson RJ (1986) The individual cell in phytoplankton ecology: Cell cycles and applications of flow cytometry. Can J Fish Aquat Sci Bull 214:343–369
Cuhel RL, Waterbury JB (1984) Biochemical composition and short term nutrient incorporation patterns in a unicellular marine cyanobacterium, Synechococcus (WH 7803). Limnol Oceanogr 29:370–374
Doolittle WF (1979) The cyanobacterial genome, its expression and the control of that expression. Adv Microbial Physiol 20:1–102
Glover H (1985) The physiology and ecology of the marine cyanobacterial genus Synechococcus. Adv Aquat Microbiol 3:49–107
Glover H, Morris I (1981) Photosynthetic characteristics of coccoid marine cyanobacteria. Arch Microbiol 129:42–46
Glover H, Smith AE (1988) Diel pattern of carbon incorporation into biochemical constituents of Synechococcus spp. and larger algae in the Northwest Atlantic Ocean. Mar Biol 97:259–267
Glover H, Campbell L, Prezelin BB (1986) Contribution of Synechococcus spp. to size fractionated primary productivity in three water masses in the Northwest Atlantic Ocean. Mar Biol 91:193–203
Glover H, Prezelin BB, Campbell L, Wyman M, Garside C (1988) A nitrate-dependent Synechococcus bloom in surface Sargasso Sea water. Nature 331:161–163
Guillard RRL, Ryther JH (1962) Studies of marine planktonic diatoms. I. Cyclotella nana and Detonula confervacea. Can J Microbiol 8:229–239
Hanson RS, Phillips JA (1981) Chemical composition. In: Gerhardt P (ed) Manual of methods for general bacteriology. American Society Microbiology, Washington, DC, pp 328–364
Hayashi F, Ishida MR, Kikuchi T (1969) Macromolecular synthesis in a blue green alga, Anacystic nidulans, in dark and light phases. Annu Rep Res React Inst Tokyo Univ 2:56–66
Herdman M, Janvier M, Rippka R, Stanier RY (1979) Genome size of cyanobacteria. J Gen Microbiol 111:73–85
Hutchinson WC, Munro HN (1961) The determination of nucleic acid in biological materials. Analyst 86:768–813
Ingraham JL, Maaloe O, Neidhardt FC (1983) Growth of the bacterial cell. Sinauer Assoc., Sunderland, MA
Jinks-Robertson S, Gourse RL, Nomura M (1983) Expression of rRNA and tRNA genes in Escherichia coli: Evidence for feedback regulation by products of rRNA operons. Cell 33:865–876
Johnson PW, Seiburth J McN (1979) Chroococcoid cyanobacteria in the sea: A ubiquitous and diverse phototrophic biomass. Limnol Oceanogr 24:928–935
Koch AL (1971) The adaptive response of Escherichia coli to a feast and famine existence. Adv Microbial Physiol 6:147–217
Kramer JG (1988) Macromolecular bases of growth regulation in marine Synechococcus spp. Unpublished PhD Dissertation, The University of Maryland, College Park Maryland
Kramer JG (1990) The effects of irradiance and specific inhibitors on protein and nucleic acid synthesis in the marine cyanobacterium Synechococcus sp. WH 7803. Arch Microbiol 154:280–285
Lin RI-S, Schjeide OA (1969) Micro estimation of RNA by the cupic ion catalyzed orcinol reaction. Anal Biochem 27:473–483
Maaloe O (1979) Regulation of the protein synthesizing machinery: Ribosomes, tRNA, factors and so on. In: Goldberger RF (ed) Biological regulation and development, vol I. Plenum Press, New York, pp 487–542
Maaloe O, Kjeldegaard NO (1966) Control of macromolecular synthesis. Benjamin, New York
Mann NH (1974) The control of growth and cell division in the blue green alga Anacystis nidulans. Unpublished PhD Thesis, University of Liverpool
Mann NH, Carr NG (1974) Control of macromolecular composition and cell division in the blue green alga Anacystic nidulans. J Gen Microbiol 83:399–405
Mann NH, Carr NG, Midgley JEM (1975) RNA synthesis and the accumulation of guanine nucleotides during growth shift down in the blue green alga Anacystis nidulans. Biochim Biophys Acta 402:41–50
Morris I, Glover H (1981) Physiology of photosynthesis by marine coccoid cyanobacteria; some ecological implications. Limnol Oceanogr 26:957–961
Newman J, Wyman M, Carr NG (1987) Absence of the nitrogen reserve polymer cyanophycin from marine Synechococcus species. FEMS Microbiol Lett 44:221–224
Nierlich DP (1974) Regulation of bacterial growth. Science 184:1043–1049
Nierlich DP (1978) Regulation of bacterial growth, RNA and protein synthesis. Annu Rev Microbiol 32:393–432
Nomura M, Gourse R, Baughman G (1984) Regulation of the synthesis of ribosomes and ribosomal components. Annu Rev Biochem 53:75–117
Olson RJ, Chisolm SW, Zettler ER, Armbrust EV (1988) Analysis of Synechococcus pigment types in the sea using single and double beam flow cytometry. Deep Sea Res 35:425–440
Parrot LM, Slater JH (1980) The DNA, RNA and protein composition of the cyanobacterium Anacystis nidulans grown in light and carbon dioxide limited chemostats. Arch Microbiol 127:53–58
Paul JH, Meyers B (1982) Fluorometric determination of DNA in aquatic microorganisms by use of Hoechst 33258. Appl Environ Microbiol 43:1393–1399
Platt T, Subba Rao DV, Irwin B (1983) Photosynthesis of picoplankton in the oligotrophic ocean. Nature 301:702–704
Prezelin BB, Putt M, Glover HE (1986) Diurnal patterns in photosynthetic capacity and depth-dependent photosynthesis-irradiance relationship in Synechococcus spp. and larger phytoplankton in three water masses in the Northwest Atlantic Ocean. Mar Biol 91:205–217
Prezelin BB, Glover HE, Campbell L (1987) Effects of light intensity and nutrient availability on diel patterns of cell metabolism and growth in populations of Synechococcus spp. Mar Biol 95:469–480
Roberts TM, Klotz LC, Loeblich AR (1977) Characterization of a blue-green algal genome. J Mol Biol 110:341–361
Smith RJ, Carr NG (1977) The regulation of stable RNA synthesis in the blue green alga Anacystis nidulans. The effect of leucine deprivation and 5-methyltryptophan inhibition. J Gen Microbiol 103:61–68
Takahashi M, Bienfang PK (1983) Size structure of phytoplankton biomass and photosynthesis in subtropical Hawaiian water. Mar Biol 76:203–211
Utkilen HC (1982) Magnesium limited growth of the cyanobacterium Anacystis nidulans. J Gen Microbiol 128:1849–1862
Utkilen HC (1984) Growth and macromolecular composition of Anacystis nidulans at different temperatures in Mg2+ and K+-limited chemostats. Arch Microbiol 138:244–246
Utkilen HC, Briseid T (1984) The response of Mg2+ limited Anacystis nidulans to alterations in photon fluence rate when grown in chemostats: a suitable system for studies of Mg2+-controlled cell division. J Gen Microbiol 130:3079–3083
Waterbury JB, Watson SW, Guillard RRL, Brand LE (1979) Widespread occurrence of a unicellular marine planktonic cyanobacterium. Nature 277:293–294
Waterbury JB, Watson SW, Valois FW, Franks DG (1987) Biological and ecological characterization of the marine unicellular cyanobacterium Synechococcus. Can J Fish Aquat Sci Bull 214:71–120
Wyman M, Gregory RPF, Carr NG (1985) A novel role for phycoerythrin in marine cyanobacterium, Synechococcus sp. DC2. Science 230:818–820
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kramer, J.G., Morris, I. Growth regulation in irradiance limited marine Synechococcus sp. WH 7803. Arch. Microbiol. 154, 286–293 (1990). https://doi.org/10.1007/BF00248969
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00248969