Abstract
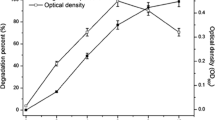
The metabolism of phenanthrene, a polycyclic aromatic hydrocarbon (PAH), by Streptomyces flavovirens was investigated. When grown for 72 h in tryptone yeast extract broth saturated with phenanthrene, the actinomycete oxidized 21.3% of the hydrocarbon at the K-region to form trans-9,10-dihydroxy-9,10-dihydrophenanthrene (phenanthrene trans-9,10-dihydrodiol). A trace of 9-phenanthrol was also detected. Metabolites isolated by thin-layer and high performance liquid chromatography were identified by comparing chromatographic, mass spectral, and nuclear magnetic resonance properties with those of authentic compounds. Experiments using [9-14C]phenanthrene showed that the trans-9,10-dihydrodiol had 62.8% of the radioactivity found in the metabolites. Circular dichroism spectra of the phenanthrene trans-9,10-dihydrodiol indicated that the absolute configuration of the predominant enantiomer was (−)-9S,10S, the same as that of the principal enantiomer produced by mammalian enzymes. Incubation of S. flavovirens with phenanthrene is an atmosphere of 18O2, followed by gas chromatographic/mass spectral analysis of the metabolites, indicated that one atom from molecular oxygen was incorporated into each molecule of the phenanthrene trans-9,10-dihydrodiol. Cytochrome P-450 was detected in 105,000×g supernatants prepared from cell extracts of S. flavovirens. The results show that the oxidation of phenanthrene by S. flavovirens was both regio- and stereospecific.
Similar content being viewed by others
Abbreviations
- CD:
-
circular dichroism
- DMF:
-
N,N-dimethyl-formamide
- GC/MS:
-
gas chromatography/mass spectrometry
- HPLC:
-
high performance liquid chromatography
- NMR:
-
nuclear magnetic resonance
- ODS:
-
octadecylsilane
- PAH:
-
polycyclic aromatic hydrocarbon
- TLC:
-
thin-layer chromatography
- TMS:
-
tetramethylsilane
- UV:
-
ultraviolet
References
Balani SK, Van Bladeren PJ, Shirai N, Jerina DM (1986) Resolution and absolute configuration of K-region trans dihydrodiols from polycyclic aromatic hydrocarbons. J Org Chem 51:1773–1778
Black JA, Birge WJ, Westerman AG, Francis PC (1983) Comparative aquatic toxicology of aromatic hydrocarbons. Fundam Appl Toxicol 3:353–358
Boyland E, Sims P (1962) Metabolism of polycyclic compounds. 21. The metabolism of phenanthrene in rabbits and rats: Dihydrodihydroxy compounds and related glucosiduronic acids. Biochem J 84:571–582
Boyland E, Wolf G (1950) Metabolism of polycyclic compounds. 6. Conversion of phenanthrene into dihydroxydihydrophenanthrenes. Biochem J 47:64–69
Bruice PY, Bruice TC, Dansette PM, Selander HG, Yagi H, Jerina DM (1976) Comparison of the mechanisms of solvolysis and rearrangement of K-region vs. non-K-region arene oxides of phenanthrene. Comparative solvolytic rate constants of K-region and non-K-region arene oxides. J Am Chem Soc 98:2965–2973
Bücker M, Glatt HR, Platt KL, Avnir D, Ittah Y, Blum J, Oesch F (1979) Mutagenicity of phenanthrene and phenanthrene K-region derivatives. Mutat Res 66:337–348
Cerniglia CE (1984) Microbial metabolism of polycyclic aromatic hydrocarbons. Adv Appl Microbiol 30:31–71
Cerniglia CE, Heitkamp MA (1989) Microbial degradation of polycyclic aromatic hydrocarbons (PAH) in the aquatic environment. In: Varanasi U (ed) Metabolism of polycyclic aromatic hydrocarbons in the aquatic environment. CRC Press, Boca Raton, Florida, pp 41–68
Cerniglia CE, Yang SK (1984) Stereoselective metabolism of anthracene and phenanthrene by the fungus Cunninghamella elegans. Appl Environ Microbiol 47:119–124
Cerniglia CE, Campbell WL, Freeman JP, Evans FE (1989) Identification of a novel metabolite in phenanthrene metabolism by the fungus Cunninghamella elegans. Appl Environ Microbiol 55:2275–2279
Chaturapit S, Holder GM (1978) Studies on the hepatic microsomal metabolism of [14C]phenanthrene. Biochem Pharmacol 27: 1865–1871
Colla C, Fiecchi A, Treccani V (1959) Ricerche sul metabolismo ossidativo microbico dell'antracene e del fenantrene. Nota II: Isolamento e caratterizzazione del 3,4-diidro-3,4-diossifenantrene. Ann Microbiol Enzimol 9:87–91
Crawford DL, Sutherland JB (1979) The role of actinomycetes in the decomposition of lignocellulose. Dev Ind Microbiol 20:143–151
Evans WC, Fernley HN, Griffiths E (1965) Oxidative metabolism of phenanthrene and anthracene by soil pseudomonads: The ring-fission mechanism. Biochem J 95:819–831
Gibson DT, Subramanian V (1984) Microbial degradation of aromatic hydrocarbons. In: Gibson DT (ed) Microbial degradation of organic compounds. Marcel Dekker, New York, pp 181–252
Grossbard E (1971) The utilization and translocation by microorganisms of carbon-14 derived from the decomposition of plant residues in soil. J Gen Microbiol 66:339–348
Guerin WF (1989) Phenanthrene degradation by estuarine surface microlayer and bulk water microbial populations. Microb Ecol 17:89–104
Harvey RG, Goh SH, Cortez C (1975) “K-region” oxides and related oxidized metabolites of carcinogenic aromatic hydrocarbons. J Am Chem Soc 97:3468–3479
Heitkamp MA, Cerniglia CE (1987) Effects of chemical structure and exposure on the microbial degradation of polycyclic aromatic hydrocarbons in freshwater and estuarine ecosystems. Environ Toxicol Chem 6:535–546
Heitkamp MA, Freeman JP, Miller DW, Cerniglia CE (1988) Pyrene degradation by a Mycobacterium sp.: Identification of ring oxidation and ring fission products. Appl Environ Microbiol 54:2556–2565
Jerina DM, Selander H, Yagi H, Wells MC, Davey JF, Mahadevan V, Gibson DT (1976) Dihydrodiols from anthracene and phenanthrene. J Am Chem Soc 98:5988–5996
Kiyohara H, Nagao K, Nomi R (1976) Degradation of phenanthrene through o-phthalate by an Aeromonas sp. Agric Biol Chem 40:1075–1082
Kusk KO (1981) Comparison of the effects of aromatic hydrocarbons on a laboratory alga and natural phytoplankton. Bot Mar 24:611–613
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Mansour FA, Shady MA (1984) Factors influencing the activity and production of nitrate reductase by certain local isolates of Streptomyces. Egypt J Bot 27:105–117
Miura R, Honmaru S, Nakazaki M (1968) The absolute configurations of the metabolites of naphthalene and phenanthrene in mammalian systems. Tetrahedron Lett 50:5271–5274
O'Keefe DP, Romesser JA, Leto KJ (1988) Identification of constitutive and herbicide inducible cytochromes P-450 in Streptomyces griseolus. Arch Microbiol 149:406–412
Omura T, Sato R (1964) The carbon monoxide-binding pigment of liver microsomes. I. Evidence for its hemoprotein nature. J Biol Chem 239:2370–2378
Pipe RK, Moore MN (1986a) An ultrastructural study on the effects of phenanthrene on lysosomal membranes and distribution of the lysosomal enzyme-β-glucuronidase in digestive cells of the periwinkle Littorina littorea. Aquat Toxicol 8:65–76
Pipe RK, Moore MN (1986b) Arylsulphatase activity associated with phenanthrene induced digestive cell deletion in the marine mussel Mytilus edulis. Histochem J 18:557–564
Rafii F, Crawford DL (1988) Transfer of conjugative plasmids and mobilization of a nonconjugative plasmid between Streptomyces strains on agar and in soil. Appl Environ Microbiol 54:1334–1340
Rogoff MH, Wender I (1957) The microbiology of coal. I. Bacterial oxidation of phenanthrene. J Bacteriol 73:264–268
Romesser JA, O'Keefe DP (1986) Induction of cytochrome P-450-dependent sulfonylurea metabolism in Streptomyces griseolus. Biochem Biophys Res Commun 140:650–659
Sariaslani FS, Kunz DA (1986) Induction of cytochrome P-450 in Streptomyces griseus by soybean flour. Biochem Biophys Res Commun 141:405–410
Savino JF, Tanabe LL (1989) Sublethal effects of phenanthrene, nicotine, and pinane on Daphnia pulex. Bull Environ Contam Toxicol 42:778–784
Shiaris MP (1989) Seasonal biotransformation of naphthalene, phenanthrene, and benzo[a]pyrene in surficial estuarine sediments. Appl Environ Microbiol 55:1391–1399
Smith RV, Rosazza JP (1974) Microbial models of mammalian metabolism. Aromatic hydroxylation. Arch Biochem Biophys 161:551–558
Solbakken JE, Palmork KH (1981) Metabolism of phenanthrene in various marine animals. Comp Biochem Physiol 70C:21–26
Stevenson PM, Ruettinger RT, Fulco AJ (1983) Cytochrome P-450 revealed: The effect of the respiratory cytochromes on the spectrum of bacterial cytochrome P-450. Biochem Biophys Res Commun 112:927–934
Sutherland JB (1986) Demethylation of veratrole by cytochrome P-450 in Streptomyces setonii. Appl Environ Microbiol 52:98–100
Sutherland JB, Blanchette RA, Crawford DL, Pometto AL (1979) Breakdown of Douglas-fir phloem by a lignocellulose-degrading Streptomyces. Curr Microbiol 2:123–126
Sutherland JB, Crawford DL, Pometto AL (1981) Catabolism of substituted benzoic acids by Streptomyces species. Appl Environ Microbiol 41:442–448
Tausson WO (1928) Die Oxidation des Phenanthrens durch Bakterien. Planta 5:239–273
Tomaszewski JE, Jerina DM, Daly JW (1975) Deuterium isotope effects during formation of phenols by hepatic monooxygenases. Evidence for an alternative to the arene oxide pathway. Biochemistry 14:2024–2031
Trower MK, Sariaslani FS, Kitson FG (1988) Xenobiotic oxidation by cytochrome P-450-enriched extracts of Streptomyces griseus. Biochem Biophys Res Commun 157:1417–1422
Yoshikawa T, Ruhr LP, Flory W, Giamalva D, Church DF, Pryor WA (1985) Toxicity of polycyclic aromatic hydrocarbons. I. Effect of phenanthrene, pyrene, and their ozonized products on blood chemistry in rats. Toxicol Appl Pharmacol 79:218–226
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sutherland, J.B., Freeman, J.P., Selby, A.L. et al. Stereoselective formation of a K-region dihydrodiol from phenanthrene by Streptomyces flavovirens . Arch. Microbiol. 154, 260–266 (1990). https://doi.org/10.1007/BF00248965
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00248965