Abstract
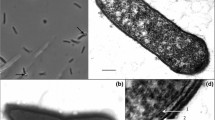
A gram positive, motile rod-shaped strictly anaerobic non sporulating bacterium was isolated from an enrichment initiated with mullet gut contents. The organism grew optimally at 30°C at pH 6.5 and at a salinity of 10/103. Out of a variety of mono-, di-, and polysaccharides tested only pectin, cellobiose and starch actively supported growth in either semi defined medium or peptone-yeast extract (PY) medium. Galacturonic acid and maltose were less effective as substrates. Mol product per 100 mol of pectin monomer degraded were: acetate, 163; ethanol, 30; methanol, 88 and formate, 48. Per 100 mol of hexose in cellobiose or starch degraded, the amounts were acetate, 39; ethanol, 128 and formate, 41. Hydrogen was not detectable in the incubations (detection limit, <10-5 atm) and propionate, butyrate, lactate or succinate were not produced as fermentation end-products (<2 mol per 100 mol monomer). The guanine plus cytosine content of DNA from the bacterium was 31 mol%, and the cell walls contained meso-diaminopimelic acid. A phylogenetic analysis of the organism by 16S rDNA sequencing and DNA-DNA homology indicated that the organism grouped more closely with several species of Clostridium than with Eubacterium. The phenotypic characteristics of the organism indicated that it did not fit within the genus Clostridium and more closely resembled Eubacterium. The organism is therefore designated as a species of Eubacterium; the type strain is P-1 (DSM 6788).
Similar content being viewed by others
References
Anderson AJ, Green RS, Starmann AJ, Archibald AR (1978) Cell wall assembly in Bacillus subtilis: location of wall material incorporated during pulsed release of phosphate limitation, its accessibility to bacteriophages and Concanavalin A, and its susceptibility to turnover. J Bacteriol 136: 886–899
Avrova NP (1975) Synthesis of pectolytic enzymes by Clostridium felsineum and their hydrolysis of the pectin substances of flax straw. Appl Biochem Microbiol 11: 736–741
Bailey RW (1958) The reaction of pentoses with anthrone. Biochem J 68: 669–672
Balch WE, Wolfe RS (1976) New approach to the cultivation of methanogenic bacteria: 2-mercaptoethane sulfonic acid (HS-CoM) dependent growth of Methanobacterium ruminantium in a pressurized atmosphere. Appl. Environ Microbiol 32: 781–791
Bauchop T, Mountfort DO (1981) Cellulose fermentation by a rumen anaerobic fungus in both the absence and presence of rumen methanogens. Appl Environ Microbiol 42: 1103–1110
Blumenkrantz N, Asboe-Hansen G (1973) New method for quantitative determination of uronic acids. Anal Biochem 54: 484–489
Bryant MP (1972) Commentary on the Hungate technique for culture of anaerobic bacteria. Am J Clin Nutr 25: 1324–1328
Cato EP, Stackebrandt E (1989) Taxonomy and phylogeny. In: Minton NP, Clarke DJ (eds) Clostridia. Plenum Press, New York London, pp 1–26
DeSoete G (1983) A least squares algorithm for fitting additive trees to proximity data. Psychometrika 48: 621–626
Duncan CL, Strong DH (1968) Improved medium for sporulation of Clostridium perfringens. Appl Microbiol 16: 82–89
Holdeman LV, Cato EP, Moore WEC (1977) Anaerobic laboratory manual. 4th edn. Anaerobe Laboratory, Virgina Polytechnic Institute and State University, Blacksburg, p 130
Hungate RE (1969) A roll-tube method for the cultivation of strict anaerobes. In: Norris JR, Ribbons DE (eds) Methods in microbiology, vol 3B. Academic Press, London, pp 117–132
Jukes TH, Cantor CR (1969) Evolution of protein molecules. In: Munro HN (ed) Mammalian protein metabolism. Academic Press, New York, pp 21–132
Jusic D, Roy C, Schocher AJ, Watson RW (1963) Chromatographic separation of isomeric dinitrophenyl derivatives of alpha-epsilon diaminopimelic acid. Can J Biochem Physiol 41: 817–820
Kertesz ZJ (1951) The pectic substances. Interscience Publishers, New York
King JD, White DC (1977) Muramic acid as a measure of microbial biomass in estuarine and marine samples. Appl Environ Microbiol 33: 777–783
Lund BM, Brocklehurst TE (1978) Pectic enzymes of pigmented strains of Clostridium. J Gen Microbiol 104: 59–66
MacDonald NL, Stark JR, Austin B (1986) Bacterial microflora in the gastro-intestinal tract of Dover Sole (Solea g solea L.), with emphasis on the possible role of bacteria in the nutrition of the host. FEMS Microbiol Lett 35: 107–111
Maniatis T, Fritsch CR, Sambrook J (1982) Molecular cloning. A laboratory manual. Cold Spring Harbor Laboratory Cold Spring Harbor, NY
Moore WEC, Holdeman-Moore LV (1986) Genus Eubacterium (Prevot 1938) In: Sneath PHA, Mair NS, Sharpe ME, Holt JG (eds) Bergey's manual of systematic bacteriology, vol 2. Williams and Wilkins, Baltimore, pp 1353–1373
Mountfort DO, Rhodes L (1991) Anaerobic growth and fermentation characteristics of Paecilomyces lilacinus isolated from mullet gut. Appl Environ Microbiol 57: 1973–1968
Mountfort DO, Grant WD, Clarke R, Asher RA (1988) Eubacterium callanderi sp nov. that demethoxylates o-methoxylated aromatic acids to volatile fatty acids. Int J Syst Bacteriol 38: 254–258
Patel BKC, Morgan HW, Daniel RM (1985) Fervidobacterium nodosum gen. nov. and spec. nov., a new chemoorganotrophic, caldactive anaerobic bacterium. Arch Microbiol 141: 63–69
Pereival E, McDowell RH (1967) Chemistry and enzymology of marine algal polysaccharides. Academic Press, London New York
Prescott GW (1968) The Algae: a review. Houghton Mifflin Co. Addison-Westey, Reading Mass, pp 8–39
Rainey FA, Dorsch M, Morgan HW, Stackebrandt E (1992) 16S rDNA analysis of Spirochaeta thermophila: its phylogenetic position and implications for the systematics of the order Spirochaetales. Syst Appl Microbiol 15: 197–202
Rexová-Benková L, Markovic O (1976) Pectic enzymes. Adv Carbohydr Chem Biochem 33: 323–385
Rhuland LE, Work E, Denman RF, Hoare DS (1955) The behavior of the isomers of α,ε-diaminopimelic acid on paper chromatograms. J Am Chem Soc 77: 4844–4846
Sakata T, Okabayas J, Kakimoto D (1980) Variations in the intestinal microflora of Tilapia reared in fresh and seawater. Bull Jap Soc Scient Fish 46: 313–317
Schink B, Zeikus JG (1982) Microbial ecology of pectin decomposition in anoxic lake sediments. J Gen Microbiol 128: 393–404
Schink B, Zeikus JG (1983) Clostridium thermosulfurogenes sp. nov., a new thermophile that produces elemental sulfur from thiosulfate. J Gen Microbiol 129: 1149–1158
Schleifer KH, Kandler O (1972) Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev 36: 407–477
Tanner RS, Stackebrandt E, Fox GE, Woese CR (1981) A phylogenetic analysis of Acetobacterium woodii, Clostridium barkeri, Clostridium lituseburense, Eubacterium limosum and Eubacterium tenue. Curr Microbiol 5: 35–38
VanRijssel M, Hansen TA (1989) Fermentation of pectin by a newly isolated Clostridium thermosaccharolyticum strain. FEMS Microbiol Lett 61: 41–46
Voragen AGJ, Schols HA, Vries JA de, Pilnik W (1982) High performance liquid chromatographic analysis of uronic acids and oligogalacturonic acids. J Chromatogr 244: 327–336
Weizenegger M, Neumann M, Stackebrandt E, Weiss N, Ludwig W (1992) Eubacterium alactolyticum phylogenetically groups with Eubacterium limosum, Acetobacterium woodii and Clostridium barkeri. Syst Appl Microbiol 15: 32–36
Zeikus JG, Hegge PW, Thompson TE, Phelps TJ, Langworthy TA (1983) Isolation and description of Haloanaerobium praevalens gen nov. and sp. nov. an obligately anaerobic halophile common to great salt lake sediments. Curr Microbiol 9: 225–234
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mountfort, D.O., Grant, W.D., Morgan, H. et al. Isolation and characterization of an obligately anaerobic, pectinolytic, member of the genus Eubacterium from mullet gut. Arch. Microbiol. 159, 289–295 (1993). https://doi.org/10.1007/BF00248486
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00248486