Abstract
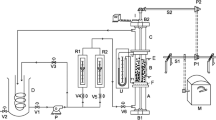
Rates of electropolishing of vertical copper cylinders with active ends in H3PO4 were studied by measuring the limiting current under natural convection. Variables studied were H3PO4 concentration, cylinder diameter and aspect ratio. The rate of polishing of the whole cylinder was represented by the mass transfer equation
for the range 1.17 × 1010 < Sc Gr < 5.11 × 1011. Rates of mass transfer were measured also at vertical cylinder with insulated ends, and the upward facing surface (disc). Data for the vertical cylindrical surface were represented for the range 8.75 × 109 < Sc Gr < 1.1 × 1012 by the equation
while data at the upward facing disc were correlated for the range 0.11 × 1010 < Sc Gr < 46 × 1010 by the equation
A comparison between the measured rate of mass transfer at the whole cylinder and the value calculated by adding the rates of mass transfer at the separate surfaces of the cylinder shows that the measured value deviates from the calculated value, the degree of deviation increases with increasing Sc × Gr. Deviation was attributed to flow interaction at the different cylinder surfaces.
Similar content being viewed by others
Abbreviations
- A :
-
anode area
- C :
-
concentration of Cu2+
- C s :
-
saturation solubility of copper phosphate (as Cu2+) in H3PO4
- C H :
-
concentration of H+
- C A :
-
concentration of anion H2PO −4
- D :
-
diffusivity of Cu2+
- D H :
-
diffusivity of H+
- D A :
-
diffusivity of H2PO −4
- d :
-
cylinder diameter
- E :
-
potential
- F :
-
Faraday constant
- g :
-
acceleration due to gravity
- I :
-
limiting current
- i :
-
limiting current density
- K :
-
mass transfer coefficient
- L :
-
cylinder height
- L c :
-
characteristic length calculated from Equation 10
- N Cu :
-
flux of Cu2+
- N H :
-
flux of H+
- NA :
-
flux of H2PO −4
- R :
-
gas constant
- T :
-
absolute temperature
- X :
-
distance from the electrode surface
- Z :
-
number of electrons involved in the reaction
- Gr :
-
Grashof number (gL 33 3(ρi - ρb)/ν 2av ςi
- S c :
-
Schmidt number, ν/D
- Sh :
-
Sherwood number, KL c/D
- ρi :
-
interfacial density of the solution
- ρb :
-
bulk density of the solution
- νav :
-
average kinematic viscosity
- δ:
-
diffusion layer thickness
References
G. H. Sedahmed and I. Nirdosh, Z. Metallkde. 82 (1991) 243.
A. M. Ahmed and G. H. Sedahmed, J. Mater. Sci. Lett. 9 (1990) 270.
G. H. Sedahmed and L. W. Shemilt, Surf. Technol. 27 (1986) 279.
Idem, ibid. 27 (1986) 335.
C. B. Shin and D. J. Economou, Int. J. Heat Mass Transf. 33 (1990) 2191.
B. G. Ateya and H. W. Pickering, J. Appl. Electrochem. 11 (1981) 453.
J. Krysa and A. A. Wragg, ibid. 22 (1992) 429.
K. Kontturi and D. J. Schiffrin, ibid. 19 (1989) 76.
I. Nirdosh and G. H. Sedahmed, ibid. 21 (1990) 651.
G. H. Sedahmed, M. Z. El-Abd, I. A. S. Mansour, A. M. Ahmed and A. A. Wragg, ibid. 9 (1979) 1.
S. H. Glarum and J. H. Marshall, J. Electrochem. Soc. 132 (1985) 2872.
J. S. Newman, ‘Electrochemical Systems’, Prentice Hall, Englewood Cliffs, NJ (1973), p.358.
A. Vogel, ‘Textbook of Quantitative Chemical Analysis’, 5th edn, Longman, London (1989), p. 276.
M. E. Weber, P. Austraukas and S. Petsalis, Can. J. Chem. Eng. 62 (1984) 68.
C. R. Wilke, M. Eisenberg and C. W. Tobias, J. Electrochem. Soc. 100 (1953) 513.
A. A. Wragg, J. Appl. Electrochem. 21 (1991) 1047.
J. R. Selman and J. Tavakoli-Attar, J. Electrochem. Soc. 127 (1980) 1049.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sedahmed, G.H., Nirdosh, I. Mass transfer study of the electropolishing of vertical cylinders with active ends under natural convection conditions. J Appl Electrochem 25, 149–154 (1995). https://doi.org/10.1007/BF00248172
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00248172