Abstract
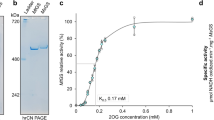
Glutamine synthetase (GS) was purified to electrophoretic homogeneity from the halophilic archaebacterium Halobacterium salinarium. The enzyme was purified 300-fold to homogeneity with 30% yield. By gel filtration and SDS gel electrophoresis, it was shown that the enzyme has a native molecular weight of 495,000 and a subunit molecular weight of 62,000. This indicates an octameric quaternary structure. The amino acid composition and the isoelectric point of 4.9 are similar to other GSs. The enzyme shows highest stability in 4 M NaCl or KCl and at temperatures up to 45°C. Lower salt concentrations or higher temperatures lead to rapid and irreversible denaturation. By low concentrations of Mg2+ or Mn2+, the salt dependence was decreased and the thermostability increased. Mg2+ or Mn2+ are essential cofactors. The two resulting activities show differences in pH and salt concentrations required for optimal activity, different K m-values and different sensitivity to inhibition by amino acids. The enzyme is not adenylylated like the GS from some eubacteria but cytidylylated. The covalently bound CMP increases Mn2+-and Mg2+-dependent activities at a different extent.
Similar content being viewed by others
References
Bhatnagar L, Zeikus JG, Aubert JP (1986) Purification and characterization of glutamine synthetase from the archaebacterium Methanobacterium ivanovi. J Bacteriol 165: 638–643
Blanco F, Alana A, Llama MJ, Serra JL (1989) Purification and properties of glutamine synthetase from the non-N2-fixing cyanobacterium Phormidium laminosum. J Bacteriol 171: 1158–1165
Danson MJ, Black SC, Woodland DL, Wood PA (1985) Citric acid cycle enzymes of the archaebacteria: citrate synthase and succinate thiokinase. FEBS Lett 179: 120–124
DeMedicis E, Paquette J, Gauthier JJ, Shapcott D (1986) Magnesium and manganese content of halophilic bacteria. Appl Environ Microbiol 52: 567–573
Guilian GG, Moss RL, Greaser M (1984) Analytical isoelectric focussing using a high-voltage vertical slab polyacrylamide gel system. Anal Biochem 142: 421–436
Hecht K, Wrba A, Jaenicke R (1989) Catalytic properties of thermophylic lactate dehydrogenase and halophilic malate dehydrogenase at high temperature and low water activity. Eur J Biochem 183: 69–74
Kingdon HS, Stadtman ER (1967) Regulation of glutamine synthetase. X. Effect of growth conditions on the susceptibility of Escherichia coli glutamine synthetase of feedback inhibition. J Bacteriol 94: 949–957
Kodama F, Fukui K, Kometani K (1986) The initial phosphate burst in ATP hydrolysis by myosin and subfragment-1 as studied by a modified malachite green method for determination of inorganic phosphate. J Biochem 99: 1465–1472
Laemmli VK (1970) Cleavage of structural proteins during the assembly of the head of the bacteriophage T4. Nature 227: 680–685
Lanyi JK (1974) Salt-dependent properties of proteins from extremly halophilic bacteria. Bacteriol Rev 38: 272–290
Levin Ö (1971) Column chromatography of proteins: calcium phosphate. In: Jacoby WB (ed) Methods in enzymology, vol 22. Academic Press, New York London, pp 325–339
Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275
Mecke D, Wulff K, Lies K, Holzer H (1966) Characterization of a glutamine synthetase inactivating enzyme from Escherichia coli. Biochem Biophys Res Commun 24: 452–458
Meister A (1974) Glutamine synthetase of mammals. In: Boyer PD (ed) The enzymes, vol 10. Academic Press, New York London, pp 699–754
Möllering H, Bergmeyer HU (1974) Adenosin. In: Bergmeyer HU (ed) Methoden der enzymatischen Analyse, 2. Auflg., vol 2. Verlag Chemie, Weinheim, pp 1967–1970
Mohr V, Larsen H (1963) On the structural transformations and lysis of Halobacterium salinarium in hypotonic and isotonic solutions. J Gen Microbiol 31: 267–280
Orr J, Keefer LM, Keim P, Nguyen TD, Wellems T, Heinrikson RL, Haselkorn R (1981) Purification, physical characterization, and NH2-terminal sequence of glutamine synthetase from the cyanobacterium Anabaena 7120. J Biol Chem 256: 13091–13098
Pundak S, Eisenberg H (1981) Structure and activity of malate dehydrogenase from the extreme halophilic bacteria from the dead sea. Eur J Biochem 118: 463–470
Reistadt R (1970) On the composition of the bulk protein of extremely halophilic bacteria. Arch Microbiol 71: 353–360
Reitzer LJ, Magasanik B (1987) Ammonia assimilation and the biosynthesis of glutamine, glutamate, aspartate, asparagine, l-alanine and d-alanine. In: Neidhard FC et al. (eds) Escherichia coli and Salmonelle typhimurium: Cellular and molecular biology, vol 1. American Society for Microbiology, Washington, pp 302–320
Ronzio RA, Rowe WB, Wilk S, Meister A (1969) Preparation and studies on the characterization of sheep brain glutamine synthetase. Biochemistry 8: 3670–2674
Segal A, Stadtman ER (1972) Variation of the conformational states of Escherichia coli glutamine synthetase by interaction with different divalent cations. Arch Biochem Biophys 152: 363–377
Shapiro BM, Kingdon HS, Stadtman ER (1967) Regulation of glutamine synthetase. VII. Adenylyl glutamine synthetase; a new form of the enzyme with altered regulatory and kinetic properties. Biochemistry 58: 642–649
Smith PK, Krohn RJ, Hermanson GT, Mallia AK, Gartner TH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150: 76–85
Stadtman ER, Ginsburg A (1974) The glutamine synthetase of Escherichia coli: structure and control. In: Boyer PD (ed) The enzymes, vol 10, Academic Press, New York London, pp 755–807
Tholey G, Bloch S, Ledig M, Mandel P, Wedler FC (1987) Chick brain glutamine synthetase and Mn2+−Mg2+ interactions. Neurochem Res 12: 1041–1047
Woese CR, Kandler O, Wheelis ML (1990) Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci USA 87: 4576–4579
Woese CR, Olson GJ (1986) Archaebacterial phylogeny: perspectives of the urkingdoms. Syst Appl Microbiol 7: 161–177
Woolfolk CA, Stadtman ER (1964) Cumulative feedback inhibition in the multiple end product regulation of glutamine synthetase activity in Escherichia coli. Biochem Biophys Res Commun 17: 313–319
Zacchai G, Cendrin F, Haik Y, Borochov N, Eisenberg H (1989) Stabilization of halophilic malate dehydrogenase. J Mol Biol 208: 491–500
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Manitz, B., Holldorf, A.W. Purification and properties of glutamine synthetase from the archaebacterium Halobacterium salinarium . Arch. Microbiol. 159, 90–97 (1993). https://doi.org/10.1007/BF00244269
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00244269