Summary
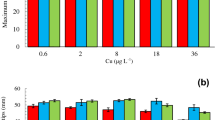
The biomass of microalgae at the bottom of first-year sea ice, in southeastern Hudson Bay (Canadian Arctic), parallels an inshore-offshore salinity gradient caused by the under-ice plume of the Great Whale River. The present study was designed to test the hypothesis that the variation of ice-algal biomass (chlorophyll a) along the salinity gradient was mainly controlled by nutrient availability, with the alternative hypothesis of a direct control by ambient salinity. The approach was that of differential in situ bioassays, conducted at the ice-water interface of two stations, located in the plume of the Great Whale River (lower salinity) and in the offshore waters of Hudson Bay (higher salinity). The inoculum (collected at the higher salinity station) was diluted with three types of seawater, i.e. (1) from the higher salinity station, (2) from the lower salinity station, and (3) from the latter but with salinity artificially increased to the level of the higher salinity station. The three sets of cultures were differentially enriched. In situ incubations for the first set were at the higher salinity station and, for the other two, at the lower salinity station. Results indicate possible Si limitation of the algal biomass at the higher salinity station. First, concentrations of Si(OH)4 observed at this station were lower than in the plume of the Great Whale River; in addition, the Si∶P molar ratios were lower than ca. 15; also, Si was the only nutrient whose addition (alone or combined with others) yielded biomasses higher than in the reference enrichment; finally, the highest growth rate for a singly added nutrient was with Si and subtraction of Si (single nutrient) was more detrimental to growth rate than that of N or P. In contrast, there was no strong indication of nutrient effects at the lower salinity station, so that nutrient limitation could not explain the lower ice-algal biomasses in lower salinity waters. At this same station, on the other hand, growth rates in water with artificially increased salinity were 2–3 times higher than those in unaltered water. These results are consistent with the hypothesis that salinity, and not nutrients, is the main factor that limits the development of ice algae in the lower salinity waters of southeastern Hudson Bay.
Similar content being viewed by others
References
Barlow RG, Gosselin M, Legendre L, Therriault JC, Demers S, Mantoura RFC, Llewellyn CA (1988) Photoadaptive strategies in sea-ice microalgae. Mar Ecol Prog Ser 45:145–152
Brown LM (1982) Photosynthetic and growth response to salinity in a marine isolate of Nannochloris bacillaris (Chlorophyceae). J Phycol 18:483–488
Conover WJ (1980) Practical nonparametric statistics. Wiley and Sons, Toronto, 493 pp
Demers S, Legendre L, Maestrini SY, Rochet M, Ingram RG (1989) Nitrogenous nutrition of sea-ice microalgae. Polar Biol 9:377–383
Gessner F, Schramm W (1971) Salinity. Plants. In: Kinne O (ed) Marine ecology, vol 1, part 2. Wiley-Interscience, London, pp 705–820
Gosselin M, Legendre L, Demers S, Ingram RG (1985) Responses of sea-ice microalgae to climatic and fortnightly tidal energy inputs (Manitounuk Sound, Hudson Bay). Can J Fish Aquat Sci 42:999–1006
Gosselin M, Legendre L, Terriault JC, Demers S, Rochet M (1986) Physical control of the horizontal patchiness of sea-ice microalgae. Mar Ecol Prog Ser 29:289–298
Gosselin M, Legendre L, Therriault JC, Demers S (1990) Light and nutrient limitation of sea-ice microalgae (Hudson Bay, Canadian Arctic). J Phycol 26:220–232
Grant WS, Horner RA (1976) Growth responses to salinity variations in four Arctic ice diatoms. J Phycol 12:180–185
Hellebust JA (1976) Osmoregulation. Ann Rev Plant Physiol 27:485–505
Hellebust JA (1978) Uptake of organic substrates by Cyclotella cryptica (Bacillariophyceae): effects of ions, ionophores and metabolic and transport inhibitors. J Phycol 14:79–83
Hellebust JA (1985) Mechanisms of response to salinity in halotolerant microalgae. Plant Soil 89:69–81
Hecky RE, Kilham P (1988) Nutrient limitation of phytoplankton in freshwater and marine environments: A review of recent evidence on the effects of enrichment. Limnol Oceanogr 33:796–822
Ingram RG, Larouche P (1987) Variability of an underice river plume in Hudson Bay. J Geophys Res 92:9541–9548
Ingram RG, Osler J, Legendre L (1989) Influence of internal wave induced vertical mixing on ice algal production in a highly stratified sound. Est Coast Shelf Sci 29:435–446
Laliberté G, Hellebust JA (1989) Regulation of proline content of Chlorella autotrophica in response to change in salinity. Can J Bot 67:1959–1965
Legendre L, Gosselin M (1991) In situ spectroradiometric estimation of microalgal biomass in first-year sea ice. Polar Biol 11:113–115
Lepage S, Ingram RG (1991) Variation of upper layer dynamics during breakup of the seasonal ice cover in Hudson Bay. J Geophys Res 96:12711–12724
Lorenzen CJ (1966) A method for the continuous measurement of in vivo chlorophyll concentration. Deep-Sea Res 13:223–227
Lund JWG, Kipling C, Le Cren ED (1958) The inverted microscope method of estimating algal numbers and the statistical basis of estimations by counting. Hydrobiologia 11:143–170
Maestrini SY, Rochet M, Legendre L, Demers S (1986) Nutrient limitation of the bottom-ice microalgal biomass (southeastern Hudson Bay, Canadian Arctic). Limnol Oceanogr 31:969–982
Michel C, Legendre L, Demers S, Therriault JC (1988) Photoadaptation of sea-ice microalgae in springtime: photosynthesis and carboxylating enzymes. Mar Ecol Prog Ser 50:177–185
Morris AW, Mantoura RFC, Bale AJ, Howland RJM (1978) Very low salinity regions of estuaries: important sites for chemical and biological reactions. Nature 274:678–680
Parsons TR, Maita Y, Lalli CM (1984) A manual of chemical and biological methods for seawater analysis. Pergamon Press, Toronto, 173 pp
Poulin M, Cardinal A, Legendre L (1983) Réponse d'une communauté de diatomées de glace à un gradient de salinité (baie d'Hudson). Mar Biol 76:191–202
Richards FA (1958) Dissolved silicate and related properties of some western North Atlantic and Caribbean waters. J Mar Res 17:449–465
Rochet M, Legendre L, Demers S (1985) Acclimation of sea-ice microalgae to freezing temperature. Mar Ecol Prog Ser 24:187–191
Stumm W, Brauner PA (1975) Chemical speciation. In: Riley JP, Skirrow G (eds) Chemical oceanography, vol 1. Academic Press, pp. 173–239
Therriault JC, Legendre L, Demers S (1990) Oceanography and ecology of phytoplankton in the St. Lawrence Estuary. In: El-Sabh M, Silverberg N (eds) Oceanography of a large-scale estuarine system. The St Lawrence. Springer, New York, pp. 269–295
Wheeler PA (1980) Use of methylammonium as an ammonium analog in nitrogen transport and assimilation studies with Cyclotella cryptica (Bacillariophyceae). J Phycol 16:328–334
Yancey PH, Clarck ME, Hand SC, Bowlus RD, Somero GN (1982) Living with water stress: evolution of osmolyte system. Science 217:1214–1222
Author information
Authors and Affiliations
Additional information
Contribution to the programs of GIROQ (Groupe interuniversitaire de recherches océanographiques du Québec) and of the Maurice Lamontagne Institute (Biological Oceanography Division, Department of Fisheries and Oceans)
Rights and permissions
About this article
Cite this article
Legendre, L., Martineau, MJ., Therriault, JC. et al. Chlorophyll a biomass and growth of sea-ice microalgae along a salinity gradient (southeastern Hudson Bay, Canadian Arctic). Polar Biol 12, 445–453 (1992). https://doi.org/10.1007/BF00243115
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00243115