Abstract
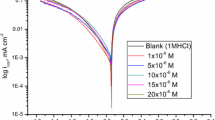
The electrochemical behaviour of aluminium covered by a thin ‘spontaneous’ oxide film was studied as a function of temperature and electrolyte composition at pH 1.3 The rectification mechanism of the charge transfer reactions is discussed on the basis of E/I characteristics. Tafel plots for the hydrogen evolution reaction showed anomalous slopes between 2.3 × 3RT/F and 2.3 × 4RT/F, depending on temperature. The inhibiting effects of 1 and 2-naphthylamines on the corrosion kinetics of aluminium in acid media (HClO4 and NaCl + HCl) were determined using electrochemical methods. Consideration of the protonation of amines and the effects of the positively charged surface suggest that the observed excellent inhibition is due to the planar orientation (with π-electron bonds) of the adsorbed inhibitor molecules and the existence of synergetic effects. The degree of surface coverage was found to increase with temperature up to 40°C. At approximately 40°C the adsorbed molecules probably change their orientation becoming vertically adsorbed on the surface with strong lateral repulsion.
Similar content being viewed by others
References
J. Gruberger and E. Gileadi, Electrochim. Acta 31 (1986) 1531.
J. W. Diggleand A. K. Vijh, ‘Oxides and Oxide Films’, Vol. 4, Marcel Dekkar, New York (1976) p. 171.
F. Qvari, L. Tomcsanyi and T. Turmezey, Electrochim. Acta 33 (1988) 323.
H. H. Streblow, Werkst. Korros. 29 (1978) 654.
J. D. Talati and D. K. Gandhi, Corros. Sci. 23 (1983) 1315.
W. Kautek, ibid. 28 (1988) 173.
T. Mimani, S. M. Mayanna and N. Munichandraiah, J. Appl. Electrochem. 23 (1993) 339.
I. L. Rozenfeld, ‘Corrosion Inhibitors’, MacGraw-Hill; New York (1981) p. 182.
M. N. Desai, B. C. Thakar, P. M. Chiaya and M. H. Gandi Corros. Sci. 16 (1976) 9.
N. Hackerman and A. C. Makrides, Ind. Eng. Chem. 46 (1954) 523.
S. L. Granese and B. M. Rosales, Proc. 7th European Symp. Corrosion Inhibitors, Ann. Univ. Ferrara, N.S., Sez. V, Suppl. N. 9, (1990) p. 73.
N. Hackerman and H. Kaesche, J. Electrochem. Soc. 105 (1958) 191.
S. M. Hassan, M. N. Mousa, F. I. Taha and A. S. Fouda, Corros. Sci. 21 (1981) 439.
A.I. Onuchukwu and F. K. Oppong-Boachie, ibid. 26 (1986) 919.
D. D. N. Singh, M. M. Singh, R. S. Chaudhary and C. V. Agarwal, Electrochim. Acta 26 (1981) 1051.
L. Homer and K. Maisel, Werkst. Korros. 29 (1978) 654.
M. Tkaicec, Ph.D. thesis, University of Zagreb, Zagreb (1977).
S. Sato, Y. Itoi and A. Hasumi, Electrochim. Acta 26 (1981) 1303.
J. O'M. Bockris and A. K. N. Reddy, ‘Modern Electrochemistry’, Vol. 2, Plenum Press, New York (1972) p. 883.
K. B. Oldham and F. Mansfeld, Corros. Sci. 11 (1971) 787, and Corrosion 27 (1971) 434.
R. E. Meyer, J. Electrochem. Soc. 107 (1960) 847.
H. J. Miao and D. L. Piron, Electrochim. Acta 38 (1993) 1079.
A. K. Vijh, J. Phys. Chem. 73 (1969) 506.
A. Despic and V. P. Parkhutik, in ‘Modern Aspects of Electrochemistry’, Vol. 20, (edited by J. O'M. Bockris, R. E. White and B. E. Conway), Plenum Press, New York (1989) p. 401.
M. Porbaix, ‘Lectures on Electrochemical Corrosion’, Plenum Press, New York (1973) p. 168.
G. A. Parks, Chem. Rev. 65 (1965) 177.
J. A. Yopps and D. W. Fuerstenau, J. Colloid Sci. 19 (1964) 61.
M. Elboujdani, E. Ghali, R. G. Barradas and M. Girgis, Corros. Sci. 30 (1990) 855.
A. Rauscher, G. Kutsan, Z. Lukacs and E. Kalman, Proc. 7th European Symp. Corrosion Inhibitors, Ann. Univ. Ferrara, N.S., Sez V, Suppl. N. 9 (1990) p. 293.
B. G. Ateya, B. E. Anadouli and F. M. Nizamy, Corros. Sci. 24 (1984) 497, 509.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Metikos-Hukovic, M., Babić, R., Grubać, Z. et al. Inhibition of the hydrogen evolution reaction on aluminium covered by ‘spontaneous’ oxide. J Appl Electrochem 24, 325–331 (1994). https://doi.org/10.1007/BF00242061
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00242061