Abstract
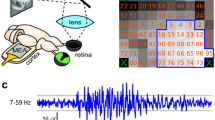
Field potentials were recorded from cat striate cortex, either between an epidural screw electrode and a cannula-electrode inserted deep in the gray matter (transcortical recording) or with a pair of metal microelectrodes. Electrodes were placed bilaterally near the cortical projection of the area centralis. The horizontal separation of the recording tips was ∼2 mm and ∼300 μm, respectively. The area of the visual field providing input to the recording site (receptive field) was determined by measuring the field potentials generated by contrast reversal of high-contrast, achromatic bar gratings. Five-degree-diameter grating patches were presented individually over a large area of the visual field. The gratings were contrast-reversed at 4, 6 or 10 Hz, while also being swept in spatial frequency between 0.56 and 5.24 c/deg. The receptive fields were ∼20 deg across or more, substantially larger than expected on the basis of cortical retinotopy. Responses were also elicited by stimulation of the hemi-field contralateral to that contributing to the classical receptive field, implicating the presence of a callosal projection. The large, spatially distributed receptive fields consisted of patches of high and low sensitivity. Continuous cortical infusion of either 100 μM tetrodotoxin or 10 mM muscimol at the recording site totally suppressed the transcortically recorded field potentials, proving that the local field potentials were generated postsynaptically. The present findings suggest that a cluster of cortical cells near the projection site of the area centralis receives input from remote cortical regions to an extent that is comparable with that of anatomically demonstrated long-range lateral connections.
Similar content being viewed by others
References
Callaway EM, Katz LC (1990) Emergence and refinement of clustered horizontal connections in cat striate cortex. J Neurosci 10:1134–1153
Cowey A (1964) Projection of the retina on to striate and prestriate cortex in the squirrel monkey, Saimiri sciureus. J Neurophysiol 27:366–393
Creutzfeldt OD, Rosina A, Ito M, Probst W (1969) Visual evoked response of single cells and of the EEG in primary visual area of the cat. J Neurophysiol 32:127–139
Doty RW (1958) Potentials evoked in cat cerebral cortex by diffuse and by punctiform photic stimuli. J Neurophysiol 21:437–464
Ebersole JS, Kaplan BJ (1981) Intracortical evoked potentials of cats elicited by punctate visual stimuli in receptive field peripheries. Brain Res 224:160–164
Fernald R, Chase R (1981) An improved method for plotting retinal landmarks and focusing the eyes. Vision Res 11:95–96
Fetz E, Toyama K, Smith W (1991) Synaptic interactions between cortical neurons. In: Peters A (ed) Cerebral cortex, vol 9. Plenum, New York, pp 1–47
Gilbert CD (1983) Microcircuitry of the visual cortex. Ann Rev Neurosci 6:217–247
Gilbert CD, Wiesel TN (1979) Morphology and intracortical projections of functionally characterized neurones in the cat visual cortex. Nature 280:120–125
Gilbert CD, Wiesel TN (1983) Clustered intrinsic connections in cat visual cortex. J Neurosci 3:1116–1133
Gilbert CD, Wiesel TN (1989) Columnar specificity of intrinsic horizontal and corticocortical connections in cat visual cortex. J Neurosci 9:2432–2442
Gilbert CD, Hirsch JA, Wiesel TN (1990) Lateral interactions in visual cortex. In: Cold Spring Harbor Symp Quant Biol 55:663–677
Harris FJ (1978) On the use of windows for harmonic analysis with the discrete Fourier transform. Proc IEEE 66:51–83
Hata Y, Tsumoto T, Sato H, Tamura H (1991) Horizontal interactions between visual cortical neurons studied by cross-correlation analysis in the cat. J Physiol (Lond) 441:593–614
Hill DR, Bowery NG (1981) 3H-Baclofen and 3H-GABA bind to bicuculline-insensitive GABAb sites in rat brain. Nature 290:149–152
Hirsch J, Gilbert CD (1991) Synaptic physiology of horizontal connections in the cat's visual cortex. J Neurosci 11:1800–1809
Hubel DH, Wiesel TN (1962) Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol (Lond) 160:106–154
Hubel DH, Wiesel TN (1974) Uniformity of monkey striate cortex: a parallel relationship between size, scatter, and magnification factor. J Comp Neurol 158:295–306
Hubel DH, Wiesel TN (1977) Functional architecture of monkey visual cortex. Proc R Soc Lond [Biol] 198:1–59
Kasamstsu T, Kitano M, Sutter EE, Norcia AM (1991) Intracortical interactions in cat visual cortex: evidence from postsynaptic field potentials. Soc Neurosci Abstr 17:1089
Kitano M, Kasamatsu T, Norcia AM (1990) Intracortical connectivity revealed by evoked potentials in cat visual cortex. Soc Neurosci Abstr 16:569
Kitano M, Kasamatsu T, Norcia AM (1991) Evidence for longrange lateral connections: direct comparison between single units and field potentials recorded from the same cortical loci (Abstr). Invest Ophthalmol Vis Sci 32:1036
Kitano M, Niiyama K, Kasamatsu T, Sutter EE, Norcia AM (1994) Retinotopic and non-retinotopic field potentials in cat visual cortex. Vis Neurosci 11:953–977
Leventhal AG, Hirsch HVB (1978) Receptive-field properties of neurons in different laminae of visual cortex in the cat. J Neurophysiol 41:948–962
Levick WR (1972) Another tungsten microelectrode. Med Biol Eng 10:510–515
Luhmann HJ, Singer W, Martinez-Millan L (1990) Horizontal interactions in cat striate cortex: I. Anatomical substrate and postnatal development. Eur J Neurosci 2:344–357
Mardia J (1982) Statistics of directional data. Wiley, New York
Martin JH (1982) Properties of cortical neurons, the EEG and the mechanisms of epilepsy. In: Kandel ER, Schwartz JH (eds) Principles of neural science. Elsevier North Holland, New York, pp 461–471
Martin KAC, Whitteridge D (1984) Form, function and intracortical projections of spiny neurones in striate visual cortex of the cat. J Physiol (Lond) 353:463–504
Mason A, Nicoll A, Stratford K (1991) Synaptic transmission between individual pyramidal neurons of the rat visual cortex in vitro. J Neurosci 11:72–84
Matsubara J, Cynader M, Swindale NV, Stryker MP (1985) Intrinsic projections within visual cortex: evidence for orientation specific local connections. Proc Natl Acad Sci USA 82:935–939
Matsubara JA, Cynader MS, Swindale NV (1987) Anatomical properties and physiological correlates of the intrinsic connections in cat area 18. J Neurosci 7:1428–1446
McGuire BA, Gilbert DC, Rivlin PK, Wiesel TN (1991) Targets of horizontal connections in macaque primary visual cortex. J Comp Neurol 305:370–392
McIlwain JT (1964) Receptive fields of optic tract axons and lateral geniculate cells: peripheral extent and barbiturate sensitivity. J Neurophysiol 27:1154–1173
McIlwain JT (1966) Some evidence concerning the physiological basis of the periphery effect in the cat's retina. Exp Brain Res 1:265–271
Michalski A, Gerstein GL, Czarkowska J, Tarnecki R (1983) Interctions between cat striate cortex neurons. Exp Brain Res 51:97–107
Mitzdorf U (1985) Current source-density method and application in cat cerebral cortex: investigation of evoked potentials and EEG phenomena. Physiol Rev 65:37–100
Mitzdorf U (1986) The physiological causes of VEP: current source density analysis of electrically and visually evoked potentials. In: Cracco RQ, Bodis-Wollner I (eds) Evoked potentials. Liss, New York, pp 141–154
Niiyama K, Kasamatsu T, Sutter EE, Norcia AM (1993) Response properties of geniculale cells revealed by m-sequence-modulated contrast reversal of bar gratings (Abstr). Invest Ophthatmol Vis Sci 34:792
Norcia AM, Clarke M, Tyler CW (1985) Digital filtering and robust regression techniques for estimating sensory threshold from the evoked potential. IEEE Eng Med Biol 4:26–32
Norcia AM, Tyler CW, Hamer RD, Wesemann W (1989) Measurement of spatial contrast sensilivily with the swept contrast VEP. Vision Res 29:627–637
Ohashi T, Norcia AM, Kasamatsu T, Jampolsky A (1991) Cortical recovery from effects of monocular deprivation caused by diffusion and occlusion. Brain Res 548:63–73
Rockland KS, Eund JS (1983) Intrinsic laminar lattice connections in primate visual cortex. J Comp Neurol 216:303–318
Rockland KS, Lund JS, Humphrey AE (1982) Anatomical banding of intrinsic connections in striate cortex of tree shrews (Tupaia glis). J Comp Neurol 209:41–58
Rodieck RW, Pettigrew JD, Bishop PO, Nikara T (1967) Residual eye movements in receptive-field studies of paralyzed cats. Vision Res 7:107–110
Schroeder CE, Tenke CE, Givre SJ, Arezzo JC, Vaughan Jr HG (1991) Striate cortical contribution to the surface-recorded pattern-reversal VEP in the alert monkey. Vision Res 31:1143–1157
Schwarz C, Bolz J (1991) Functional specificity of a long-range horizontal connection in cat visual cortex: a cross-correlation study. J Neurosci 11:2995–3007
Synder A, Shapley R (1979) Deficits in the visual evoked potentials of cats as a result of visual deprivation. Exp Brain Res 37:73–86
Toyama K, Kimura M, Tanaka K (1981a) Cross-correlational analysis of interneuronal connectivity in cat visual cortex. J Neurophysiol 46:191–201
Toyama K, Kimura M, Tanaka K (1981b) Organization of cat visual cortex as investigated by cross-correlational technique. J Neurophysiol 46:202–213
Ts'o DY, Gilbert CD, Wiesel TN (1986) Relationships between horizontal interactions and functional architecture in cat striate cortex as revealed by cross-correlation analysis. J Neurosci 6:1160–1170
Tusa RJ, Palmer EA, Rosenquist AC (1978) The retinotopic organization of area 17 (striate cortex) in the cat. J Comp Neurol 177:213–236
Vakkur GJ, Bishop PO, Kozak W (1963) Visual optics in the cat, including posterior nodal distance and retinal landmarks. Vision Res 3:289–314
Wong-Riley M (1979) Changes in the visual system of monocularly sutured or enucleated cats demonstrable with cytochrome oxidase histochemistry. Brain Res 17:11–28
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kitano, M., Kasamatsu, T., Norcia, A.M. et al. Spatially distributed responses induced by contrast reversal in cat visual cortex. Exp Brain Res 104, 297–309 (1995). https://doi.org/10.1007/BF00242015
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00242015