Abstract
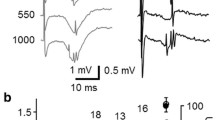
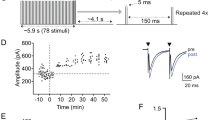
In supragranular layers of the rat auditory cortex, white matter stimulation produces antidromic and transsynaptic field potentials, of which only the latter shows long-term potentiation (LTP) following tetanic stimulation of the white matter. In this study, we investigated the cells responsible for the LTP. The transsynaptic field potentials, excitatory postsynaptic potentials (EPSPs), and orthodromic spikes were blocked by 6-cyano-7-nitroquinoxaline-2, 3-dione (10 μM), but not by d-2-amino-5-phosphonovalerate (D-AP5, 50 μM). The latency of EPSPs was constant, while that of transsynaptic field potentials and orthodromic spikes was shortened by the increase in stimulus intensity. Appearance of anti-dromic field potentials and antidromic spikes at strong stimulus intensities were accompanied by reduction in amplitude of transsynaptic field potentials and elimination of orthodromic spikes, respectively. Morphological identification of neurons showing antidromic spikes by intracellular injection of biocytin revealed that most of them were supragranular pyramidal cells. The effects of tetanic stimulation were studied by intracellular recording in seven neurons showing, antidromic spikes, and it was found that only two of them showed LTP of EPSP slope. However, in all of the other eight units showing antidromic spikes and recorded extracellularly, LTP was clearly observed in orthodromic firing probability. The LTP induction in the orthodromic firing probability was blocked by D-AP5. These findings indicate that the LTP in field potentials corresponds to LTP in supragranular pyramidal outputs, and the input-output relationship in neural networks of the adult rat auditory cortex is strongly modulated by LTP.
Similar content being viewed by others
References
Ahissar E, Vaadia E, Ahissar M, Bergman H, Arieli A, Abeles M (1992) Dependence of cortical plasticity on correlated activity of single neurons and on behavioral context. Science 257:1412–1415
Andersen P, Sundberg SH, Sveen O, Swann JW, Wigstrom H (1980) Possible mechanisms for long-lasting potentiation of synaptic transmission in hippocampal slices from guinea-pigs. J Physiol (Lond) 302:463–482
Artola A, Singer W (1987) Long-term potentiation and NMDA receptors in rat visual cortex. Nature 330:649–652
Bear MF, Press WA, Connors BW (1992) Long-term potentiation in slices of kitten visual cortex and the effects of NMDA receptor blockade. J Neurophysiol 67:841–851
Blakemore C, Cooper GF (1970) Development of the brain depends on the visual environment. Nature 228:477–478
Bliss TV, Lømo T (1973) Long-lasting potentiation of synaptic transmission in the dentate area of the anesthetized rabbit following stimulation of the perforant path. J Physiol (Lond) 232:331–356
Edeline JM, Weinberger NM (1993) Receptive field plasticity in the auditory cortex during frequency discrimination training: selective retuning independent of task difficulty. Behav Neurosci 107:82–103
Felleman DJ, Van Essen DC (1991) Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex 1:1–47
Hebb DO (1949) The organization of behavior. Wiley, New York
Hirsch HV, Spinelli DN (1970) Visual experience modifies distribution of horizontally and vertically oriented receptive fields in cats. Science 168:869–871
Hirsch JA, Gilbert CD (1993) Long-term changes in synaptic strength along specific intrinsic pathways in the cat visual cortex. J Physiol (Lond) 461:247–262
Horikawa K, Armstrong WE (1988) A versatile means of intracellular labeling: injection of biocytin and its detection with avidin conjugates. J Neurosci Methods 25:1–11
Hubel DH, Wiesel TN (1963) Receptive fields of cells in striate cortex of young, visually inexperienced kittens. J Neurophysiol 26:994–1002
Keller A, Iriki A, Asanuma H (1990a) Identification of neurons producing long-term potentiation in the cat motor cortex: intracellular recordings and labeling. J Comp Neurol 300:47–60
Keller A, Pavlides C, Asanuma H (1990b) Long-term potentiation in the cat somatosensory cortex. Neuroreport 1:49–52
Kimura E, Nishigori A, Shirokawa T, Tsumoto T (1989) Long-term potentiation and N-methyl-d-aspartate receptors in the visual cortex of young rats. J Physiol (Lond) 414:125–144
Kirkwood A, Dudek SM, Gold JT, Aizenman CD, Bear MF (1993) Common forms of synaptic plasticity in the hippocampus and neocortex in vitro. Science 260:1518–1521
Komatsu Y, Fujii K, Maeda J, Sakaguchi H, Toyama K (1988) Long-term potentiation of synaptic transmission in kitten visual cortex. J Neurophysiol 59:124–141
Krieg WJS (1946) Connections of the cerebral cortex. J Comp Neurol 84:221–275
Kudoh M, Shibuki K (1994) Long-term potentiation in the auditory cortex of adult rats. Neurosci Lett 171:21–23
Liao D, Hessler NA, Malinow R (1995) Activation of postsynaptically silent synapses during pairing-induced LTP in CA1 region of hippocampal slice. Nature 375:400–404
Lorente de Nó R (1947) A study of nerve physiology. Studies from the Rockefeller Institute for Medical Research, New York, pp 131–132
MacDonald JF, Mody I, Salter MW (1989) Regulation of N-methyl-d-aspartate receptors revealed by intracellular dialysis of murine neurons in culture. J Physiol (Lond) 414:17–34
Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A (1984) Magnesium gates glutamate-activated channels in mouse central neurons. Nature 307:462–465
Rall W (1962) Electrophysiology of a dendritic neuron model. Biophys J 2:145–167
Recanzone GH, Schreiner CE, Merzenich MM (1993) Plasticity in the frequency representation of primary auditory cortex following discrimination training in adult owl monkeys. J Neurosci 13:87–103
Toyama K, Matsunami K, Ohno T (1969) Antidromic identification of association, commissural and corticofugal efferent cells in cat visual cortex. Brain Res 14:513–517
Tsumoto T, Suda K (1979) Cross-depression: an electrophysiological manifestation of binocular competition in the developing visual cortex. Brain Res 168:190–194
Tsumoto T, Masui H, Sato H (1986) Excitatory amino acid transmitters in neuronal circuits of the cat visual cortex. J Neurophysiol 55:469–483
Watkins JC, Evans RH (1981) Excitatory amino acid transmitters. Annu Rev Pharmacol Toxicol 21:165–204
Wong D (1991) Cellular organization of the cat's auditory cortex. In: Altschuler RA, Bobbin RP, Clopton BM, Hoffman DW (eds) Neurobiology of hearing: the central auditory system. Raven, New York, pp 367–387
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kudoh, M., Shibuki, K. Long-term potentiation of supragranular pyramidal outputs in the rat auditory cortex. Exp Brain Res 110, 21–27 (1996). https://doi.org/10.1007/BF00241370
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00241370