Summary
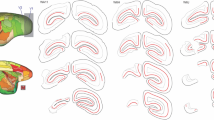
Visual callosal connections were examined using autoradiographic (ARG) and horse-radish peroxidase (HRP) techniques in normal adult hamsters, and in adults subjected to ablation of the superficial tectal laminae at birth. Additional ARG and HRP experiments were carried out in hamsters 1–27 days of age in order to describe the normal development of this pathway. Neonatal collicular lesions, which deprived visual cortical neurons of a major terminal zone in the midbrain, substantially altered the visual callosal pathway. In the lesioned animals, the numbers of supragranular callosal cells in the 17–18a border region and lamina VI callosal neurons in medial area 17 were significantly greater than normal. The ARG experiments demonstrated additional clearcut abnormalities in the visual callosal pathway of the lesioned hamsters. First, the mediolateral extent of the supragranular callosal zone around the 17–18a border was increased. Secondly, dense label was visible over lower layer V and lamina VI throughout area 17. Finally, labelling in lamina I could also be observed across the entire mediolateral extent of area 17.
Experiments in the developing hamsters suggested that some of the abnormalities observed in the lesioned animals may have resulted from the maintenance of normally transient developmental states. During the first postnatal week, both callosal cells and anterograde labelling were evenly distributed throughout the dorsal posterior neocortex, but only in the subplate region. During the second postnatal week, supragranular callosal cells were also labelled in both medial and lateral area 17, but from their first appearance, they were always most numerous in the 17–18a border region. At the same time callosal axons invaded the supragranular laminae, but only near the 17–18a border. By the end of the second postnatal week, the visual callosal pathway was very similar to that in the adult.
Similar content being viewed by others
References
Adams JC (1980) Stabilizing and rapid thionin staining of TMB-based HRP reaction product. Neurosci Lett 17: 7–9
Arnett D, Spraker TE (1981) Cross-correlation analysis of the maintained discharge of rabbit retinal ganglion cells. J Physiol (Lond) 317: 29–47
Baisinger J, Lund RD, Miller B (1977) Aberrant retinothalamic projections resulting from unilateral tectal lesions made in fetal and neonatal rats. Exp Neurol 54: 369–382
Berlucchi G (1981) Recent advances in the analysis of the neural substrates of interhemispheric communication. In: Pompeiano O, Marsan CA (eds) Brain Mechanisms and Perceptual Awareness. Raven Press, New York, pp 133–152
Burne RA, Azizi SA, Mihailoff GA, Woodward DJ (1981) The tectopontine projection in the rat with comments on visual pathways to the basilar pons. J Comp Neurol 202: 287–307
Caviness VS (1975) Architectonic map of neocortex of the normal mouse. J Comp Neurol 164: 247–264
Chow KL, Baumbach HD, Lawson R (1981) Callosal projections of the striate cortex in the neonatal rabbit. Exp Brain Res 42: 122–126
Cowan WM, Gottlieb DI, Hendrickson AE, Price JC, Woolsey TA (1972) The autoradiographic demonstration of axonal connections in the central nervous system. Brain Res 37: 21–51
Crain BJ, Hall WC (1980) The organization of the lateral posterior nucleus of the golden hamster after neonatal superior colliculus lesions. J Comp Neurol 193: 383–401
Cunningham TJ (1972) Sprouting of the optic projections after cortical lesions. Anat Rec 172: 298
Cusick CG, Lund RD (1982) Modification of visual callosal projections in rats. J Comp Neurol 212: 385–398
Devor M (1976) Neuroplasticity in the rearrangement of olfactory tract fibers after neonatal transection in hamsters. J Comp Neurol 166: 49–72
Devor M, Schneider GE (1975) Neuroanatomical plasticity: the principle of conservation of total axonal arborization. In: Vital-Durand F, Jeannerod M (eds) Aspects of Neural Plasticity. Inserm 43: 191–200
Dürsteler BC, Garey LS (1979) Projections to the visual cortex in the golden hamster. J Comp Neurol 183: 185–204
Fish SE, Rhoades RW (1981) A method for making very small, quantifiable, micropipette injections of axonal tracer substances. J Neurosci Meth 4: 291–297
Frost DO (1981) Orderly anamolous retinal projections to the medial geniculate, ventrobasal, and lateral posterior nuclei of the hamster. J Comp Neurol 203: 227–256
Holcombe V, Hall WC (1981) Laminar origin of ipsilateral tectopontine pathways. Neurosci 6: 255–260
Innocenti GM (1981) Growth and reshaping of axons in the establishment of visual callosal connections. Science 212: 824–827
Innocenti GM, Fiore L, Caminiti R (1977) Exuberant projection into the callosum from the visual cortex of newborn cats. Neurosci Lett 4: 237–242
Innocenti GM, Frost DO (1979) Abnormal visual experience stabilizes juvenile patterns of interhemispheric connections. Nature 280: 231–234
Innocenti GM, Frost DO (1980) The postnatal development of visual callosal connections in the absence of visual experience or of the eyes. Exp Brain Res 39: 365–375
Ivy GO, Akers RM, Killacky HP (1979) Differential distribution of callosal projection neurons in the neonatal and adult rat. Brain Res 173: 532–537
Ivy GO, Killackey HP (1981) The ontogeny of the distribution of callosal projection neurons in the rat parietal cortex. J Comp Neurol 195: 367–389
Jen L, Lund RD, Boles J (1978) Further studies on the aberrant crossed visual corticotectal pathway in rats. Exp Brain Res 33: 405–414
Kalil RE, Schneider GE (1975) Abnormal synaptic connections of the optic tract in the thalamus after midbrain lesions in newborn hamsters. Brain Res 100: 690–698
Kostovic I, Molliver ME (1974) A new interpretation of the laminar development of cerebral cortex: synaptogenesis in different layers of neopallium in the human fetus. Anat Rec 178: 395
Lund RD, Mitchell DE (1979a) The effects of dark rearing on visual callosal connections of cats. Brain Res 167: 172–175
Lund RD, Mitchell DE (1979b) Asymmetry in the visual caallosal connections of strabismic cats. Brain Res 167: 176–179
Lund RD, Mitchell DE, Henry GH (1978) Squint-induced modification of callosal connections in cats. Brain Res 144: 169–172
Mason R, Groos GA (1981) Cortico-recipient and tecto-recipient visual zones in the rat's lateral posterior (pulvinar) nucleus: an anatomical study. Neurosci Lett 25: 107–112
Mesulam M-M (1976) The blue reaction product in horseradish peroxidase neurohistochemistry: Incubation parameters and visibility. J Histochem Cytochem 24: 1273–1280
Mesulam M-M (1978) Tetramethyl benzidine for horseradish peroxidase neurohistochemistry: a non-carcinogenic blue reaction product with superior sensitivity for visualizing neural afferents and efferents. J Histochem Cytochem 26: 106–117
Perry VH (1980) A tectocortical visual pathway in the rat. Neurosci 5: 915–928
Perry VH, Cowey A (1979a) Changes in the retino-fugal pathways following cortical and tectal lesions in neonatal and adult rats. Exp Brain Res 35: 97–108
Perry VH, Cowey A (1979b) The effects of unilateral cortical and tectal lesions on retinal ganglion cells in rats. Exp Brain Res 35: 85–95
Rhoades RW, Chalupa LM (1978) Functional and anatomical consequences of neonatal visual cortical damage in the superior colliculus of the golden hamster. J Neurophysiol 41: 1466–1494
Rhoades RW, DellaCroce DR (1980a) Visual callosal connections in the golden hamster. Brain Res 190: 231–237
Rhoades RW, DellaCroce DR (1980b) Neonatal enucleation induces an asymmetric pattern of visual callosal connections in hamsters. Brain Res 202: 189–195
Rhoades RW, Fish SE (1983) Bilateral enucleation alters visual callosal but not corticotectal or corticogeniculate projections in hamster. Exp Brain Res 51: 451–462
Rhoades RW, Kuo DC, Polcer JD (1982) Effects of neonatal cortical lesions upon retinocollicular projections in the hamster. Neurosci 7: 2441–2458
Rhoades RW, Mooney RD, Fish SE (1982) Anatomical organization of visual cortical and superior collicular inputs to lateral posterior nucleus in hamster. Invest Ophthalmol 22: 46
Rice FL, Van der Loos H (1977) Development of the barrels and barrel field in the somatosensory cortex of the mouse. J Comp Neurol 171: 545–560
Robson JA, Hall WC (1977) The organization of the pulvinar in the grey squirrel (sciurus carolinensis) I. Cytoarchitecture and connections. J Comp Neurol 173: 355–388
Rosene DL, Mesulam M-M (1978) Fixation variables in horseradish peroxidase neurohistochemistry. I. Effects of fixation time and perfusion procedures upon enzyme activity. J Histochem Cytochem 26: 28–39
Schneider GE (1970) Mechanisms of functional recovery following lesions of visual cortex or superior colliculus in neonate and adult hamsters. Brain Behav Evol 3: 295–323
Schneider GE (1973) Early lesions of superior colliculus: factors affecting the formation of abnormal projections. Brain Behav Evol 8: 73–109
Sefton AJ, Mackay-Sim A, Baur LA, Cottee LJ (1981) Cortical projections to visual centres in the rat: An HRP study. Brain Res 215: 1–13
Shatz CJ (1977) Anatomy of interhemispheric connections in the visual system of Boston Siamese and ordinary cats. J Comp Neurol 173: 497–518
So K, Jen LS (1982) Visual callosal corticotectal and corticogeniculate projections in golden hamsters. Brain Behav Evol 21: 125–136
So K-F, Schneider GE (1978) Abnormal recrossing retinotectal projections after early lesions in Syrian hamsters: age related effects. Brain Res 147: 277–295
Stein BE, Edwards SB (1979) Corticotectal and other cortifugal projections in neonatal cat. Brain Res 161: 399–409
Udin SB (1978) Retinotectal compression in the hamster: a quantitative study with horseradish peroxidase. J Physiol (Lond) 280: 47–48p
Udin SB and Schneider GE (1981) Compressed retinotectal projection in hamsters: Fewer ganglion cells project to tectum after neonatal tectal lesions. Exp Brain Res 43: 261–269
Wiesendanger R, Wiesendanger M (1982) The corticopontine system in the rat. I. Mapping of corticopontine neurons. J Comp Neurol 208: 215–226
Wise SP, Jones EG (1976) The organization and postnatal development of the commissural projection of the somatic sensory cortex of the rat. J Comp Neurol 168: 313–343
Yorke CH, Caviness VS (1975) Interhemispheric neocortical connections of the corpus callosum in the normal mouse: A study based on anterograde and retrograde methods. J Comp Neurol 164: 233–246
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mooney, R.D., Rhoades, R.W. & Fish, S.E. Neonatal superior collicular lesions alter visual callosal development in hamster. Exp Brain Res 55, 9–25 (1984). https://doi.org/10.1007/BF00240494
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00240494