Abstract
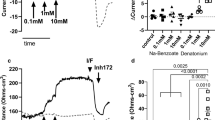
The role of tight junctions in modulating responses from chorda tympani (taste) and lingual (general sensory) nerves are clarified in regard to their responses to salts. Chorda tympani (CT) responses elicited by organic sodium salts require greater Na+ concentrations to elicit the same magnitude of response as NaCl. These data can be understood in terms of the organic anions (compared with Cl−) producing larger liquid-junction potentials across tight junctions between taste cells which, in turn, reduces Na+ influx into taste cells via amiloride-inhibitable channels. The anion contribution to the CT response to different Na+ salts can be eliminated (or enhanced) by voltage clamping the tongue with negative (with respect to the serosal solution) potentials.
Whole nerve recordings from the lingual branch of the trigeminal nerve elicited by NaCl (and other salts) were reversibly inhibited by the tight junction blocker, LaCl3 These data suggest that small hydrophilic molecules elicit responses from trigeminal fibers by diffusing across tight junctions between epithelial cells and altering the composition of the extracellular space.
Similar content being viewed by others
References
Mistretta CM: Permeability of tongue epithelium and its relation to taste. Am J Physiol 220: 1162–1167, 1971
Murray RM: Ultrastructure of taste receptors. In: L Beidler (ed.) Handbook of Sensory Physiology. Springer-Verlag, New York, 1971
Akisaka T, Oda M: Taste buds in the vallate papillae of the rat studied with freeze-fracture preparation. Arch Hist Jpn 41: 87–98, 1978
Holland VF, Zampighi GA, Simon SA: Tight junctions in taste buds: possible role in intravascular taste. Chem Senses 16: 69–79, 1991
Holland VD, Zampighi GA, Simon SA: Morphology of fungiform papillae in canine lingual epithelium: Location of intercellular junctions in the epithelium. J Comp Neurol 279: 13–27, 1989
Kruger L, Mantyh PW: Gustatory and related chemosensory systems. In: A. Bjorkland, T Hokfelt and LW Swanson (eds) Handbook of Chemical Neuroanatomy: Integrated Systems of the CNS, Part 11, Vol. 7. 1989, pp 323–411
Yamasaki H, Kubota Y, Tohyama M: Ontonogy of substance P-containing fibers in taste buds and surrounding epithelium. 1. Light microscopic analysis. Dev Brain Res 18: 301–305, 1985
Finger TE, St. Jeor VL, Kinnamon JC, Silver WL: Ultrastructure of substance P- and CGRP-immunoreactive nerve fibers in the nasal epithelium of rodents. J Comp Neurol 294: 293–305, 1990
Avenet P, Lindemann B: Amiloride-blockable sodium currents in isolated taste receptor cells. J Memb Biol 105: 245–255, 1988
Roper SD: The cell-biology of vertebrate taste receptors. Ann Rev Neurosci 12: 329–353, 1989
Schiffman SS: Contribution of the anion to the taste quality of sodium salts. In: MR Kare, MJ Fregly and RA Bernard (eds) Biological and Behavioral Aspects of Salt Intake. Academic Press, New York, 1980, pp 99–111
Beidler LM: Anion influences on taste receptor response. In: T. Hayashi (ed.) Olfaction and Taste 11. Pergamon Press, Oxford, 1967, pp 509–535
Formaker BK, Hill DL: An analysis of residual NaCl taste response after amiloride. Am J Physiol 255: R1002-R1007, 1988
Nakamura M, Kurihara K: Temperature dependence of amiloride-sensitive and -insensitive component of rat taste nerve response to NaCl. Brain Research 444: 159–164, 1988
Heck G, Persuad JA, DeSimone JA: Direct measurement of translingual epithelial NaCl and KCl currents during the chorda tympani taste response. Biophys J 55: 843–857, 1989
Sostman AL, Simon SA: Trigeminal nerve responses in rat elicited by chemical stimulation of the tongue. Archs Oral Biol 36: 95–102, 1991
Harper HW: A diffusion potential model of salt taste receptors. Annals NY Acad Sci 510: 349–351, 1987
Ye Q, Heck GL, DeSimone JA: The anion paradox in sodium taste reception: resolution by voltage-clamp studies. Science 254: 724–726, 1991
Green BG, Gelhard B: Salt as an oral irritant. Chem Senses 14: 259–271, 1989
Elliott EJ, Simon SA: The anion in salt taste: a possible role of tight junctions. Brain Res 535: 9–17, 1990
Schiffman SS, Lockhead E, Maes FW: Amiloride reduces the taste intensity of Na+ and Li+ salts. Proc Acad Sci USA 80: 6136–6140, 1983
Sostman AL, Simon SA: Trigeminal nerve responses in rat elicited by chemical stimulation of the tongue. Archs Oral Biol 36: 95–102, 1991
Hill DL, Bour TC: Addition of functional amiloride-sensitive components to the receptor membrane: a possible mechanism for altered taste responses during development. Dev Brain Res 20: 310–313, 1985
Hille B: Ionic channels of excitable membranes 1–426. Sinauer Associates Inc., Sunderland, MA, 1984
Wright EM, Diamond JM: Anion selectivity in biological systems. Physiol Rev 57: 109–156, 1977
Avenet P, Lindemann B: Perspectives of taste reception. J Membrane Biol 112: 1–8, 1989
Simon SA, Holland VF, Zampighi GA: Localization of Na-KATPase in lingual epithelia. Chem Senses 16: 283–293, 1991
Reuss L: Cell volume regulation in nonrenal epithelia. Renal Physiol Biochem 3–5: 187–201, 1988
Demarest JR, Finn AL: Characterization of the basolateral membrane conductance of Necturus urinary bladder. J Gen Physiol 89: 541–562, 1987
Palmer LG: Voltage-dependent block by amiloride and other monovalent cations of apical Na channels in the toad urinary bladder. J Membrane Biol 80: 153–165, 1984
Palmer LG: Interactions of amiloride and other blocking cations with the apical Na channel in the toad urinary bladder. J Membrane Biol 87: 191–199, 1985
Simon SA, Sostman AL: Electrophysiological responses to nonelectrolytes in lingual nerve of rat and lingual epithelia of dog. Archs Oral Biol 35: 805–813, 1991
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Simon, S.A. Influence of tight junctions on the interaction of salts with lingual epithelia: responses of chorda tympani and lingual nerves. Mol Cell Biochem 114, 43–48 (1992). https://doi.org/10.1007/BF00240296
Issue Date:
DOI: https://doi.org/10.1007/BF00240296