Summary
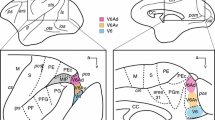
The distribution of retrogradely labeled cells in the nuclei of the vestibular nuclear complex following injections of horseradish peroxidase in various parts of the cerebellar cortex (except the nodulus and paraflocculus) has been mapped in the macacus rhesus monkey. In the main the findings correspond to those made in other mammalian species (cf. Table 1). The flocculus receives afferents bilaterally from the superior, medial and descending vestibular nuclus, group y, the interstitial nucleus of the vestibular nerve and also from the abducent nucleus. The projection to the posterior vermis (lobules VIII and IX), expecially to lobule IX, is more abundant than that to lobules VI–VII. The projection to the anterior lobe vermis appears to be modest. Evidence for projections to the cerebellar hemispheres was not obtained. Whether the lateral vestibular nucleus projects to the cerebellum in the macaque is uncertain. The regular occurrence of weakly labeled cells among heavily labeled ones suggests that many of the cerebellar projecting cells may have axonal branches passing to other destinations. The findings lend support to the notion that there are precise topical relations within the entire secondary vestibulocerebellar projection. For example, in the medial nucleus the sites of origin of fibers to the flocculus and uvula are different. Surprisingly, many cells in group z were found to project to the uvula and — to a lesser extent — to lobule VIII. The group z may, therefore, not be a pure relay nucleus in a spinothalamic pathway, as generally assumed. The rather marked cerebellar projection of the abducent nucleus, expecially to the flocculus, is of interest for the analysis of cerebellar control of eye movements in the macaque.
Similar content being viewed by others
References
Ahlsén G (1981) Retrograde labelling of retinogeniculate neurones in the cat by HRP uptake from the diffuse injection zone. Brain Res 223: 374–380
Alley K, Baker R, Simpson JI (1975) Afferents to the vestibulocerebellum and the origin of the visual climbing fibers in the rabbit. Brain Res 98: 582–589
Batini C, Corvisier J, Hardy O, Jassick-Gerschenfeld D (1977) Perihypoglossal and secondary vestibular projections to lobules VI and VII of the cerebellar cortex: an HRP study. Neurosci Lett 5: 111–116
Blanks RHI, Precht W, Torigoe Y (1983) Afferent projections to the cerebellar flocculus in the pigmented rat demonstrated by retrograde transport of horseradish peroxidase. Exp Brain Res 52: 293–306
Brodal A (1940) Modification of Gudden method for study of cerebral localization. Arch Neurol Psychiat (Chicago) 43: 46–58
Brodal A (1983) The perihypoglossal nuclei in the macaque monkey and the chimpanzee. J Comp Neurol 218: 257–269
Brodal A (1984) The vestibular nuclei in the macaque monkey. J Comp Neurol 227: 252–266
Brodal A, Angaut P (1967) The termination of spinovestibular fibres in the cat. Brain Res 5: 494–500
Brodal A, Brodal P (1983) Observations on the projection from the perihypoglossal nuclei onto the cerebellum in the macaque monkey. Arch Ital Biol 121: 151–166
Brodal A, Pompeiano O (1957) The vestibular nuclei in the cat. J Anat (Lond) 91: 438–454
Brodal A, Torvik A (1957) Über den Ursprung der sekundären vestibulo-cerebellaren Fasern bei der Katze. Eine experimentell-anatomische Studie. Arch Psychiat Nervenkr 195: 550–567
Brodal P (1979) The pontocerebellar projection in the rhesus monkey: an experimental study with retrograde axonal transport of horseradish peroxidase. Neuroscience 4: 193–208
Brodal P (1982) Further observations on the cerebellar projections from the pontine nuclei and the nucleus reticularis tegmenti pontis in the rhesus monkey. J Comp Neurol 204: 44–55
Brodal P, Brodal A (1981) The olivocerebellar projection in the monkey. Experimental studies with the method of retrograde tracing of horseradish peroxidase. J Comp Neurol 201: 375–393
Brodal P, Brodal A (1982) Further observations on the olivocerebellar projection in the monkey. Exp Brain Res 45: 71–83
Carleton SC, Carpenter MB (1983) Afferent and efferent connections of the medial, inferior and lateral vestibular nuclei in the cat and monkey. Brain Res 278: 29–51
Carpenter MB (1960) Experimental anatomical-physiological studies of the vestibular nerve and cerebellar connections. In: Rasmussen GL, Windle W (eds) Neural mechanisms of the auditory and vestibular systems. Thomas CC, Springfield, Ill, pp 297–323
Cohen B (1974) The vestibulo-ocular reflex arc. In: Kornhuber HH (ed) Handbook of sensory physiology, Vol VI/1. Springer, Berlin Heidelberg New York, pp 477–540
Colman DR, Scalia F, Cabrales E (1976) Light and electron microscopic observations on the anterograde transport of horseradish peroxidase in the optic pathway in the mouse and rat. Brain Res 102: 156–163
Coulter JD, Mergner T, Pompeiano O (1976) Effects of static tilt on cervical spinoreticular tract neurons. J Neurophysiol 39: 45–62
Dow RS (1936) The fiber connections of the posterior parts of the cerebellum in the rat and cat. J Comp Neurol 63: 527–548
Edwards SB (1972) The ascending and descending projections of the red nucleus in the cat: an experimental study using an autoradiographic tracing method. Brain Res 48: 45–63
Frankfurter A, Weber JT, Harting JK (1977) Brain stem projections to lobule VII of the posterior vermis in the squirrel monkey: as demonstrated by the retrograde axonal transport of tritiated horseradish peroxidase. Brain Res 124: 135–139
Gacek RR (1977) Location of brain stem neurons projecting to the oculomotor nucleus in the cat. Exp Neurol 57: 725–749
Goldberg YM, Fernández C (1980) Efferent vestibular system in the squirrel monkey: anatomical location and influence of afferent activity. J Neurophysiol 43: 986–1025
Gordon G, Grant G (1982) Dorsolateral spinal afferents to some medullary sensory nuclei. Exp Brain Res 46: 12–23
Gould BB (1980) Organization of afferents from the brain stem nuclei to the cerebellar cortex in the cat. Adv Anat Embryol Cell Biol 62: 1–90
Gould BB, Graybiel AM (1976) Afferents to the cerebellar cortex in the cat: evidence for an intrinsic pathway leading from the deep nuclei to the cortex. Brain Res 110: 601–611
Grant G, Boivie J, Silfvenius H (1973) Course and termination of fibres from the nucleus z of the medulla oblongata. An experimental light microscopical study in the cat. Brain Res 55: 55–70
Graybiel AM (1977a) Direct and indirect preoculomotor pathways of the brainstem: an autoradiographic study of the pontine reticular formation in the cat. J Comp Neurol 175: 37–78
Graybiel AM (1977b) Organization of oculomotor pathways in the cat and Rhesus monkey. In: Baker R, Berthoz A (eds) Control of gaze by brain stem neurons, Developments in neuroscience, Vol 1. Elsevier/North-Holland, Amsterdam, pp 79–88
Graybiel AM, Hartwieg EA (1974) Some afferent connections of the oculomotor complex in the cat. Brain Res 81: 543–551
Henkel CK, Martin GF (1977) The vestibular complex of the American opossum, Didelphis virginiana. II. Afferent and efferent connections. J Comp Neurol 172: 321–348
Ingvar S (1918) Zur Phylo- und Ontogenese des Kleinhirns nebst einem Versuche zu einheitlicher Erklärung der zerebellaren Funktion und Lokalisation. Folia Neuro-Biol (Lpz) 11: 205–495
Isu N, Yokota J (1983) Morphophysiological study on the divergent projection of axon collaterals of medial vestibular nucleus neurons in the cat. Exp Brain Res 53: 151–162
Ito M (1982) Cerebellar control of the vestibulo-ocular reflex — around the flocculus hypothesis. Ann Rev Neurosci 5: 275–296
Jansen J, Brodal A (1942) Experimental studies on the intrinsic fibers of the cerebellum. III. The corticonuclear projection in the rabbit and the monkey. Avh Norske Vid-Akad I Mat-Nat K1 3: 1–50
Kimoto Y, Satoh K, Sakumoto T, Tohyama M, Shimizu N (1978) Afferent fiber connections from the lower brain stem to the rat cerebellum by the horseradish peroxidase method combined with MAO staining, with special reference to noradrenergic neurons. J Hirnforsch 19: 85–100
Kotchabhakdi N, Rinvik E, Walberg F, Yingcharoen K (1980) The vestibulothalamic projections in the cat studied by retrograde axonal transport of horseradish peroxidase. Exp Brain Res 40: 405–418
Kotchabhakdi N, Walberg F (1977) Cerebellar afferents from neurons in motor nuclei of cranial nerves demonstrated by retrograde axonal transport of horseradish peroxidase. Brain Res 137: 158–163
Kotchabhakdi N, Walberg F (1978) Cerebellar afferent projections from the vestibular nuclei in the cat: An experimental study with the method of retrograde axonal transport of horseradish peroxidase. Exp Brain Res 31: 591–604
Kubin L, Magherini PC, Manzoni D, Pompeiano O (1980) Responses of lateral reticular neurons to sinusoidal stimulation of labyrinth receptors in decerebrate cat. J Neurophysiol 44: 922–936
Landgren S, Silfvenius H (1969) Projection to cerebral cortex of group I muscle afferents from the cat's hind limb. J Physiol (Lond) 200: 353–372
Landgren S, Silfvenius H (1971) Nucleus Z, the medullary relay in the projection path to the cerebral cortex of group I muscle afferents from the cat's hind limb. J Physiol (Lond) 218: 551–571
Larsell O (1970) The comparative anatomy and histology of the cerebellum from monotremes through apes. Jansen J (ed) University of Minnesota Press, Minneapolis, 269 pp
Magherini PC, Pompeiano O, Seguin JJ (1975) Responses of nucleus Z neurons to vibration of hindlimb extensor muscles in the decerebrate cat. Arch Ital Biol 113: 150–187
McCrea RA, Yoshida K, Berthoz A, Baker R (1980) Eye movement related activity and morphology of second order vestibular neurons terminating in the cat abducens nucleus. Exp Brain Res 40: 468–473
Mesulam M-M (1978) Tetramethyl benzidine for horseradish peroxidase neurohistochemistry: a non-carcinogenic blue reaction-product with superior sensitivity for visualizing neural afferents and efferents. J Histochem Cytochem 26: 106–117
Mitsacos A, Reisine H, Highstein SM (1983a) The superior vestibular nucleus: An intracellular HRP study in the cat. I. Vestibuloocular neurons. J Comp Neurol 215: 78–91
Mitsacos A, Reisine H, Highstein SM (1983b) The superior vestibular nucleus: An intracellular HRP study in the cat. II. Non-vestibulo-ocular neurons. J Comp Neurol 215: 92–107
Nijensohn DE, Kerr FWL (1975) The ascending projections of the dorsolateral funiculus of the spinal cord in the primate. J Comp Neurol 161: 459–470
Pompeiano O, Brodal A (1957) Spino-vestibular fibers in the cat. An experimental study. J Comp Neurol 108: 353–382
Precht W, Volkind R, Blanks RHI (1977) Functional organization of the vestibular input to the anterior and posterior cerebellar vermis of cat. Exp Brain Res 27: 143–160
Rubertone JA, Haines DE (1981) Secondary vestibulocerebellar projections to flocculonodular lobe in a prosimian primate, Galago senegalensis. J Comp Neurol 200: 255–272
Rubertone JA, Haines DE (1982) The vestibular complex in a prosimian primate (Galago senegalensis): Morphology and spinovestibular connections. Brain Behav Evol 20: 129–155
Ruggiero D, Batton III RR, Jayaraman A, Carpenter MB (1977) Brain stem afferents to the fastigial nucleus in the cat demonstrated by transport of horseradish peroxidase. J Comp Neurol 172: 189–210
Sadjadpour K, Brodal A (1968) The vestibular nuclei in man. A morphological study in the light of experimental findings in the cat. J Hirnforsch 10: 299–323
Shinnar S, Maciewicz RJ, Shofer RJ (1975) A raphe projection to cat cerebellar cortex. Brain Res 97: 139–143
Suzuki DA, Keller EL (1982) Vestibular signals in the posterior vermis of the alert monkey cerebellum. Exp Brain Res 47: 145–147
Voogd J, Bigaré F (1980) Topographical distribution of olivary and cortico nuclear fibers in the cerebellum: A review. In: Courville J, de Montigny C, Lamarre Y (eds) The inferior olivary nucleus: Anatomy and physiology. Raven Press, New York, pp 207–234
Walberg F, Brodal A, Hoddevik GH (1976) A note on the method of retrograde transport of horseradish peroxidase as a tool in studies of afferent cerebellar connections, particularly those from the inferior olive; with comments on the orthograde transport in Purkinje cell axons. Exp Brain Res 24: 383–401
Yamamoto M (1978) Localization of rabbit's flocculus Purkinje cells projecting to the cerebellar lateral nucleus and the nucleus prepositus hypoglossi investigated by means of the horseradish peroxidase retrograde axonal transport. Neurosci Lett 7: 197–202
Yamamoto M (1979) Topographical representation in rabbit cerebeUar flocculus for various afferent inputs from the brainstem investigated by means of retrograde axonal transport of horseradish peroxidase. Neurosci Lett 12: 29–34
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Brodal, A., Brodal, P. Observations on the secondary vestibulocerebellar projections in the macaque monkey. Exp Brain Res 58, 62–74 (1985). https://doi.org/10.1007/BF00238954
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00238954