Abstract
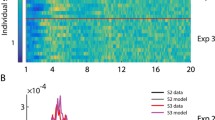
Most vertebrate animals produce optokinetic nystagmus in response to rotation of their visual surround. Nystagmus consists of an alternation of slow-phase eye rotations, which follow the surround, and fast-phase eye rotations, which quickly reset eye position. The time intervals between fast phases vary stochastically, even during optokinetic nystagmus produced by constant velocity rotation of a uniform surround. The inter-fast-phase interval distribution has a long tail, and intervals that are long relative to the mode become even more likely as constant surround velocity is decreased. This paper provides insight into fast-phase timing by showing that the process of fast-phase generation during constant velocity optokinetic nystagmus is analogous to a random walk with drift toward a threshold. Neurophysiologically, the output of vestibular nucleus neurons, which drive the slow phase, would approximate a random walk with drift because they integrate the noisy, constant surround velocity signal they receive from the visual system. Burst neurons, which fire a burst to drive the fast phase and reset the slow phase, are brought to threshold by the vestibular nucleus neurons. Such a nystagmic process produces stochastically varying inter-fast-phase intervals, and long intervals emerge naturally because, as drift rate (related to surround velocity) decreases, it becomes more likely that any random walk can meander for a long time before it crosses the threshold. The theoretical probability density function of the first threshold crossing times of random walks with drift is known to be that of an inverse Gaussian distribution. This probability density function describes well the distributions of the intervals between fast phases that were either determined experimentally, or simulated using a neurophysiologically plausible neural network model of fast-phase generation, during constant velocity optokinetic nystagmus.
Similar content being viewed by others
References
Allum JHJ, Graf W, Dichgans J, Schmidt CL (1976) Visual-vestibular interactions in the vestibular nuclei of the goldfish. Exp Brain Res 26:463–485
Balaban CD, Ariel M (1992) A ‘beat-to-beat’ interval generator for optokinetic nystagmus. Biol Cybern 66:203–216
Boyle R, Büttner U, Markert G (1985) Vestibular nuclei activity and eye movements in the alert monkey during sinusoidal optokinetic stimulation. Exp Brain Res 57:362–369
Cannon SC, Robinson DA, Shamma S (1983) A proposed neural network for the integrator of the oculomotor system. Biol Cybern 49:127–136
Chun K-S, Robinson DA (1978) A model of quick phase generation in the vestibuloocular reflex. Biol Cybern 28:209–221
Collewijn H (1975) Direction-selective units in the rabbit's nucleus of the optic tract. Brain Res 100:489–508
Curthoys IS, Nakao S, Markham CH (1981) Cat medial pontine reticular neurons related to vestibular nystagmus: firing pattern, location and projection. Brain Res 222:75–94
Easter SS (1972) Pursuit eye movements in goldfish (Carassius auratus). Vision Res 12:673–688
Galiana HL (1991) A nystagmus strategy to linearize the vestibuloocular reflex. IEEE Trans Biomed Eng 38:532–543
Galiana HL, Outerbridge JS (1984) A bilateral model for central neutral pathways in vestibuloocular reflex. J Neurophysiol 51:210–241
Hikosaka O, Maeda M, Nakao S, Shimazu H, Shinoda Y (1977) Presynaptic impulses in the abducens nucleus and their relation to postsynaptic potentials in motoneurons during vestibular nystagmus. Exp Brain Res 27:355–376
Hikosaka O, Igusa Y, Imai H (1978) Firing pattern of prepositus hypoglossi and adjacent reticular neurons related to vestibular nystagmus in the cat. Brain Res 144:395–403
Hoffmann K-P, Distler C (1989) Quantitative analysis of visual receptive fields of neurons in the nucleus of the optic tract and the dorsal terminal nucleus of the accessory optic tract in macaque monkeys. J Neurophysiol 62:416–428
Hoffmann K-P, Schoppmann A (1981) A quantitative analysis of the direction-specific response of neurons in the cat's nucleus of the optic tract. Exp Brain Res 42:146–157
Keller EL (1974) Participation of medial pontine reticular formation in eye movement generation in monkey. J Neurophysiol 37:316–332
Langer TP, Kaneko CRS (1984) Brainstem afferents to the omnipause region in the cat: a horseradish peroxidase study. J Comp Neurol 230:444–458
McCrea RA, Strassman A, May E, Highstein SM (1987) Anatomical and physiological characteristics of vastibular neurons mediating the horizontal vestibulo-ocular reflex of the squirrel monkey. J Comp Neurol 264:547–570
Nakao S, Curthoys IS, Markham CH (1980) Direct inhibitory projection of pause neurons to nystagmus-related pontomedullary reticular burst neurons in the cat. Exp Brain Res 40:283–293
Nakao S, Sasaki S, Schor RH, Shimazu H (1982) Functional organization of premotor neurons in the cat medial vestibular nucleus related to slow and fast phases of nystagmus. Exp Brain Res 45:371–385
Ohki Y, Shimazu H, Suzuki I (1988) Excitatory input to burst neurons from the labyrinth and its mediating pathway in the cat: location and functional characteristics of burster-driving neurons. Exp Brain Res 72:457–472
Remmel RS (1984) An inexpensive eye movement monitor using the scleral search coil technique. IEEE Trans Biomed Eng 31:388–390
Robinson DA (1963) A method of measuring eye movement using a scleral search coil in a magnetic field. IEEE Trans Biomed Electron 10:137–145
Robinson DA (1975) Oculomotor control signals. In: Bach-y-Rita P, Lennerstrand G (eds) Basic mechanisms of ocular motility and their clinical implications. Pergamon Press, Oxford, pp 337–374
Robinson DA (1989) Control of eye movements. In: Brooks VB (ed) Handbook of physiology, sect 1: The nervous system, vol II, part 2. American Physiological Society, Bethesda, pp 1275–1320
Sasaki S, Shimazu H (1981) Reticulo-vestibular organization participating in generation of horizontal fast eye movement. Ann NY Acad Sci 374:130–143
Schmid R, Lardini F (1976) On the predominance of anti-compensatory eye movements in vestibular nystagmus. Biol Cybern 23:135–148
Seshadri V (1993) The inverse Gaussian distribution: a case study in exponential families. Clarendon Press, Oxford
Shimazu H (1983) Neuronal organization of the premotor system controlling horizontal conjugate eye movements and vestibular nystagmus. In: Desmedt JE (ed) Motor control mechanisms in health and disease. Raven Press, New York, pp 565–588
Shimazu H, Precht W (1966) Inhibition of central vestibular neurons from the contralateral labyrinth and its mediating pathway. J Neurophysiol 29:467–492
Strassman A, Highstein SM, McCrea RA (1986a) Anatomy and physiology of saccadic burst neurons in the alert squirrel monkey. I. Excitatory burst neurons. J Comp Neurol 249:337–357
Strassman A, Highstein SM, McCrea RA (1986b) Anatomy and physiology of saccadic burst neurons in the alert squirrel monkey. II. Inhibitory burst neurons. J Comp Neurol 249:358–380
Waespe W, Henn V (1977) Neuronal activity in the vestibular nuclei of the alert monkey during vestibular and optokinetic stimulation. Exp Brain Res 27:523–538
Watanabe S, Kato I, Sato S, Norita M (1993) Direct projection from the nucleus of the optic tract to the medial vestibular nucleus in the cat. Neurosci Res 17:325–329
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Anastasio, T.J. A random walk model of fast-phase timing during optokinetic nystagmus. Biol. Cybern. 75, 1–9 (1996). https://doi.org/10.1007/BF00238734
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00238734