Abstract
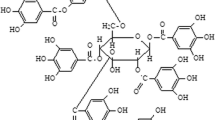
Phytic acid stimulated the myoglobin-t-butylhydroperoxide (TBHP)-catalysed oxidation of uric acid, but inhibited the peroxidation of erythrocyte membrane lipids induced by the same system. Butylated hydroxy-toluene, a free radical chain reaction-terminating antioxidant, also suppressed the myoglobin-TBHP-induced lipid peroxidation. Moreover, phytic acid inhibited the hydroxyl radical-induced degradation of deoxyribose, but the extent of inhibition in this system was reduced by increasing the ferric ion concentration, suggesting that these effects of phytic acid on the myoglobin-TBHP-mediated oxidation are more likely attributable to its metal chelating properties rather than to a free radical scavenging action. The effectiveness of phytic acid, a naturally occurring antioxidant, in the inhibition of both iron- (as previously shown) and myoglobin-dependent lipid peroxidation suggests its possible therapeutic application as a non-toxic antioxidant for ameliorating the extent of oxy-radical-mediated myocardial ischemia/reperfusion damage.
Similar content being viewed by others
Abbreviations
- ASC:
-
Ascorbic acid
- BHT:
-
Butylated Hydroxytoluene
- DMSO:
-
Dimethyl Sulfoxide
- TBHP:
-
t-Butylhydroperoxide
- TBA:
-
Thiobarbituric Acid
- TBARS:
-
Thiobarbituric Acid-reactive Substances
References
Simpson PJ, Mickelson JK, Lucchesi BR: Free radical scavengers in myocardial ischemia. Fed Proc 46: 2413–2421, 1987
Flaherty JT, Weisfeldt ML: Reperfusion injury. Free Rad Biol Med 5: 409–419, 1988
Burton KP: Evidence for direct toxic effects of free radicals on the myocardium. Free Rad Biol Med 4: 15–24, 1988
Godin DV: Role of reactive oxygen derived radicals in ischemic heart disease. Can J Cardiol 5: 235–238, 1989
Godin DV, Ko KM, Garnett ME: Altered antioxidant status in ischemic/reperfused rabbit myocardium: effects of allopurinol. Can J Cardiol 5: 365–371, 1989
Godin DV, Bhimji S, McNeill JH: Effects of allopurinol pretreatment on myocardial ultrastructure and arrhythmias following coronary artery occlusion and reperfusion. Virchows Arch. [Cell Pathol] 52 327–341, 1986
Godin DV, Bhimji S: Effects of allopurinol on myocardial ischemic injury induced by coronary artery ligation and reperfusion. Biochem Pharmacol 36: 2101–2107, 1987
Myers CL, Weiss SJ, Kirsh MM, Shepard BM, Shlafer M: Effects of supplementing hypothermic crystalloid cardioplegic solution with catalase, superoxide dismutase, allopurinol, or desferoxamine on functional recovery of globally ischemic and reperfused isolated hearts. J Thorac Cardiovasc Surg 91: 281–289, 1986
van der Kraaij AMM, van Eijk HG, Koster JF: Prevention of postischemic cardiac injury by the orally active iron chelator 1,2-dimethyl-3-hydroxyl-4-pyridine (Ll) and the antioxidant (+)-cyanidanol-3. Circulation 80: 158–164, 1989
Wittenberg JB: Myoglobin-facilitated oxygen diffusion: Role of myoglobin in oxygen entry into muscle. Physiol Rev 50: 559–636, 1970
Tajima G, Shikama K: Autoxidation of oxymyoglobin. An overall stoichiometry including subsequent side reactions. J Biol Chem 262: 12603–12605, 1987
Fox JB, Nicholas RA, Ackerman SA, Swift CE: A multiple wavelength analysis of the reaction between hydrogen peroxide and metmyoglobin. Biochemistry 13: 5178–5186, 1974
Wallace WJ, Houtchens RA, Maxwell JC, Caughey WS: Mechanism of autooxidation for hemoglobins and myoglobins. Promotion of superoxide production by protons and anions. J Biol Chem 257: 4966–4977, 1982
Arroyo CM, Kramer JH, Dickens BF, Weglicki WB: Identification of free radicals in myocardial ischemia/reperfusion by spin trapping with nitrone DMPO. FEBS Lett 221: 101–104, 1987
Bolli R, Jeroundi MO, Patel BS, DuBose CM, Lai EK, Roberts R, McCay PB: Direct evidence that oxygen-derived free radicals contribute to postischemic myocardial dysfunction in the intact dog. Proc Natl Acad Sci USA 86: 4695–4699, 1989
Kanner J, Harel S: Lipid peroxidation and oxidation of several compounds by H2O2 activated metmyoglobin. Lipids 20: 625–628, 1985
Rice HR, Lee MY, Brown WD: Interactions of heme proteins with hydrogen peroxide: protein crosslinking and co-valent binding of benzo[a]pyrene and 17-betaestradiol. Arch Biochem Biophys 221: 417–427, 1983
Graf E, Empson KL, Eaton JW: Phytic acid. A natural antioxidant. J Biol Chem 262: 11647–11650, 1987
Ko KM, Godin DV: Ferric ion-induced peroxidation of erythrocyte membrane lipids: effects of phytic acid and butylated hydroxytoluene. Mol Cell Biochem 95: 125–131, 1990.
Howell RR, Wyngaarden JB: On the mechanism of peroxidation of uric acid by hemoproteins. J Biol Chem 235: 3544–3550, 1960
Godin DV, Schrier SL: Mechanism of inactivation of adenosine triphosphate by carbodiimides. Biochemistry 9: 4068–4077, 1970
Halliwell B, Gutteridge JMC, Aruoma OI: The deoxyrihose method: A simple test-tube assay for determination of rate constants for reactions of hydroxyl radicals. Anal Biochem 165: 215–219, 1987
Millikan GA: Muscle hemoglobin. Physiol Rev 19: 503–554, 1939
Jones DP, Kennedy FG: Intracellular O2 gradients in cardiac myocytes. Lack of a role for myoglobin in facilitation of intracellular O2 diffusion. Biochem Biophys Res Commun 105: 419–424, 1982
Galaris D, Cadenas E, Hochstein P: Glutathione-dependent reduction of peroxides during ferryl- and met-myoglobin interconversion: a potential protective mechanism in muscle. Free Rad Biol Med 6: 473–478, 1989
Galaris D, Cadenas E, Hochstein P: Redox cycling of myoglobin and ascorbate: A potential protective mechanism against reperfusion injury in muscle. Arch Biochem Biophys 273: 497–504, 1989
Ames BN, Cathart R, Schwiers E, Hochstein P: Uric acid provides an antioxidant defense in humans against oxidantand radical-caused aging and cancer: A hypothesis. Proc Natl Acad Sci USA 78: 6858–6852, 1981
Davies KJA, Sevanian A, Muakkassah-Kelly SF, Hochstein P: Uric acid-iron ion complexes. A new aspect of the antioxidant functions of uric acid. Biochem J 235: 747–754, 1986
Thornalley PJ, Trotta RJ, Stern A: Free radical involvement in the oxidative phenomena induced by tert-butylhydroperoxide in erythrocytes. Biochim Biophys Acta 759: 16–22, 1983
Porter WL: Recent trends in food applications of antioxidants. In: MG Simic and M Karel (eds) Autoxidation in Food and Biology Systems. Plenum Press, New York, 1980, pp 295–366
Vincent SH, Grady RW, Shaklai N, Snider JM, Muller-Eberhard U: The influence of heme-binding proteins in heme-catalysed oxidations. Arch Biochem Biophys 265: 539–550, 1988
Martell AE: Chelates of ascorbic acid. Formation and catalytic properties. In: Ascorbic Acid: Chemistry, Metabolism and Uses. Advances in Chemistry Series 200. PA Seib and BM Tolbert (eds). Am Chem Soc, Washington, DC, pp 153–180, 1982
Brown JM, Beehler CJ, Berger EM, Grosso MA, Whitman GJ, Terada LS, Leff JA, Harken AH, Repine JE: Albumin decreases hydrogen peroxide and reperfusion injury in isolated rat hearts. Inflammation 13: 583–589, 1989
Brown JM, Grosso MA, Terada LS, Beehler CJ, Toth KM, Whitman GJ, Harken AH, Repine JE: Erythrocytes decrease myocardial hydrogen peroxide levels and reperfusion injury. Am J Physiol 256: H584-H588, 1989
Brown JM, Grosso MA, Terada LS, Whitman GJR, Banerjee A, White CW, Harken AH, Repine JE: Endotoxin pretreatment increases endogenous myocardial catalase activity and decreases ischemia-reperfusion injury of isolated rat hearts. Proc Natl Acad Sci USA 86: 2516–2520, 1989
Halliwell B: Superoxide-dependent formation of hydroxyl radicals in the presence of iron chelates: is it a mechanism for hydroxyl radical production in biochemical systems? FEBS Lett 92: 321–326, 1978
Gutteridge JMC: The role of superoxide and hydroxyl radicals in phospholipid peroxidation catalysed by iron salts. FEBS Lett 150: 454–458, 1982
Arduini A, Mezzetti A, Porreca E, Lapenna D, DeJulia J, Marzio L, Polidoro G, Cucurullo F: Effect of ischemia and reperfusion on antioxidant enzymes and mitochondrial inner membrane protein in perfused rat hearts. Biochem Biophys Acta 970: 113–121, 1988
Vallance BD, Hume R, Weyers E: Reassessment of changes in leukocyte and serum ascorbic acid after acute myocardial infarction. Br Heart J 40: 64–68, 1978
Galaris D, Eddy L, Arduini A, Cadenas E, Hochstein P: Mechanisms of reoxygenation injury in myocardial infarction: implications of a myoglobin redox cycle. Biochem Biophys Res Comm 160: 1162–1168, 1989
Kuzuya T, Hoshida S, Nishida M, Kim Y, Fuji H, Kitabatake A, Kamada T, Tada M: Role of free radicals and neutrophils in canine myocardial reperfusion injury: myocardial salvage by a novel free radical scavenger, 2-octadecylascorbic acid. Cardiovas Res 23: 323–330, 1989
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ko, K.M., Godin, D.V. Effects of phytic acid on the myoglobin-t-butylhydroperoxide-catalysed oxidation of uric acid and peroxidation of erythrocyte membrane lipids. Mol Cell Biochem 101, 23–29 (1991). https://doi.org/10.1007/BF00238434
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00238434